Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Kitchen waste is an important component of domestic waste, and it is both harmful and rich in resources. Biofuels generally refer to solid, liquid or gaseous fuels made from biological organisms and their metabolic excretions. Biofuels are pollution-free, locally available, sustainable and reliable. Biodiesel, bioalcohols (ethanol and butanol), biomethane, and biohydrogen are the typical representatives of gaseous and liquid biofuels.
- kitchen waste
- biofuel
- biogas
- biohydrogen
1. Biogas
Biogas, which contains abundant methane (approximately 60%) and carbon dioxide (approximately 40%), as well as small amounts of hydrogen sulfide, ammonia, hydrogen, and oxygen, is a common fuel. Therefore, increasing the use of biogas will alleviate the rising global energy and environmental crises [1].
Kitchen waste is partially converted into biogas through anaerobic digestion (AD) by anaerobes in the digestion pool. The production of biogas through AD is one of the best feasible options due to its low energy requirement and eco-friendly nature, in comparison with other methods [2]. The mechanism of the process is still under discussion. To date, mainly two-stage, three-stage and four-stage theories [3] have been proposed. The four-stage theory of hydrolysis→acidogenesis→acetogenesis→methanogenesis is now usually applied (Figure 1). Various anaerobic stages are induced by the corresponding anaerobic bacterial communities [4]. Clostridium, Micrococci, Bacteroides, Butyrivibrio, Fusobacterium, and Selenomonas are involved in rate-controlling steps [5], namely, they participate in the hydrolysis stage to convert insoluble organics into soluble organics.
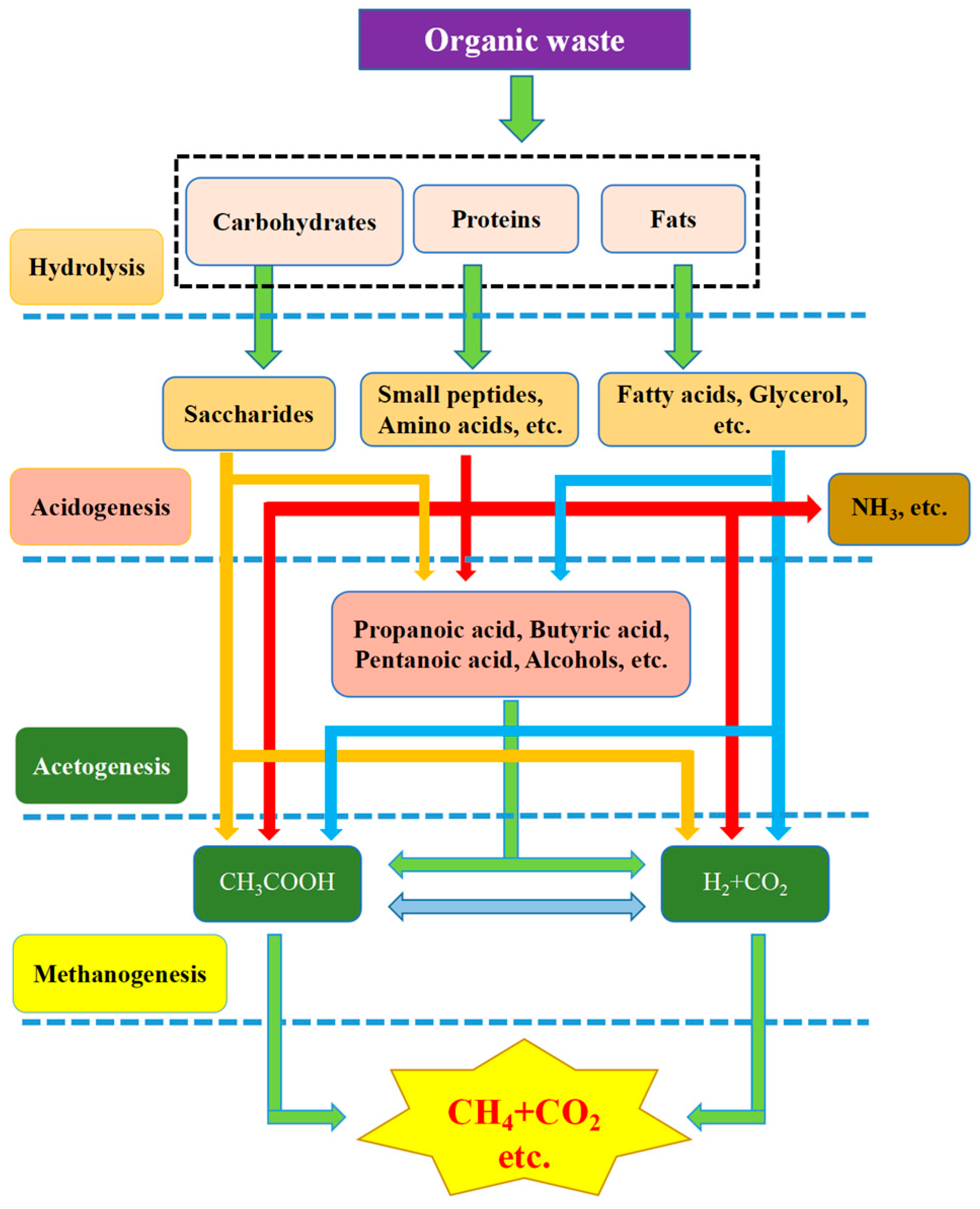
Figure 1. Four-stage theory in the anaerobic digestion of organic waste [3].
Streptococcus, Lactobacillus, Bacillus, Escherichia, and Salmonella are involved in the conversion of hydrolytic products into low molecular weight products, such as propanoic acid, butanoic acid, ethanoic acid, aliphatic acid, alcohol, and carbon dioxide. All low molecular weight products in the previous step are transformed into acetic acid by acetic acidification. The products vary with the bacterial type and cultivation environment. Methane bacteria, such as Methanomicrobiales, Methanobacteriales, and Methanococcales, are used to generate methane via acetic acid, hydrogen and carbon dioxide, which are all formed in the second and third stages [6].
Although biogas production is a relatively mature technology, it has strict technical requirements because the composition of kitchen waste is complex and variable in time and space. The anaerobic or aerobic disposal of kitchen waste is challenging due to the presence of constituents that are difficult to decompose in kitchen waste [7]. However, only 40–60% of volatile solids are available in biomethane, which indicates that pretreatment is essential. The common pretreatment methods include chemical, thermal, pressure, biological, enzymatic, and ultrasonic methods, among others [8][9][10], as described in Table 1.
Table 1. Effect of different pretreatment methods on methanogenesis of kitchen waste.
| Pretreatment | Treatment Conditions | Enhancement Effects | Possible Mechanisms | Reference |
|---|---|---|---|---|
| Thermal | Pretreatment temperatures: 80, 100, 70, 120 and 140 °C, respectively. | Cumulative biomethane production was enhanced by 22.2%, 18.9%, 9.9%, 7.5% and 3.8%, respectively. | Degradation of complex molecules, as well as the solubilization of recalcitrant particles, making the substrate more available for the anaerobes | [11] |
|
Cumulative biomethane production was enhanced by
|
A higher solubilization of COD due to various physical and microbial processes | [12] | |
|
Increasing extent of total biomethane production:
|
Both excessively low and excessively high thermal hydrolysis temperatures cannot effectively promote biogas production |
[13] | |
| A pre-heating of 30 min to 120 °C + 30 min autoclaving at 120 °C + 30 min cooling to room temperature | A 24% increase in biogas production (65% CH4) | Thermal hydrolysis disintegrates the cell membranes |
[14] | |
| Ultrasonic | Ultrasonic fequency: 20, 25 and 30 kHz; Pre-treatment time: 8, 12 and 16 min; Power density: 650, 975 and1300 W/L |
The total methane production increased by 17.87% on average and 34.48% at its peak | Destroy the structure of cell membrane and promote the release of cell contents | [15] |
| Ultrasonic strength: 250 W Treatment time: 40 min |
The cumulative gas production increased by 42.6%, and the methane concentration increased from 51.25% to 58.8% | Agitation effect to promote mixture; destroys cell wall by ultrasonic cavitation | [16] | |
| Acid | Acidified with HCl (10 N) at room temperature until pH 2, 24 h of contact time | A 48% increase in biogas production (65% CH4) | Promote the dissolution of organic matter | [14] |
| Adjusted the pH to 3.5 with HCl | The cumulative methane yield increased by 9.1% | [17] | ||
| Thermo-acid | Acidified with HCl (10 N) at room temperature until pH 2, 24 h of contact time, 30 min pre-heating to 12 °C + 30 min autoclaving at 120 °C + 30 min cooling to room temperature | A 40% increase in biogas production (65% CH4) | Promote the dissolution of organic matter | [14] |
| Pressure–depressure | Pressurized to 10 bar with CO2 as pressurizing gas. After a few minutes of contact time, the depressurization was released to 1 bar. | A 48% increase in biogas production (65% CH4) | Physically break up the microbial cell walls | [14] |
| Freeze–thaw | Frozen to 8 °C. After 6 h, the frozen KW was thawed in a thermal oven at 55 ± 2 °C for 30 min. | A 56% increase in biogas production (65% CH4) | Freezing the material at a low temperature provokes cell disruption due to intracellular ice crystals formation, causing damage to cell membranes | [14] |
| Alkali | Addition of 1% CaO | Biogas yield increases 30.67% Methane yield increases 15.49% |
The damage of chemical bonds by Ca2+ increased the ability to break down complex compounds into soluble protein, monomeric sugars and other simpler compounds | [18] |
| Bentonite | Total of 3 g/L and 5 g/L of bentonite in mesophilic and thermophilic digestion, respectively | Methane production increased by 68.52% and 56.79%, respectively | Bentonite with large surface area and porous property could provide a sufficient habitat for microorganism growth. Moreover, due to its high adsorption capacity and constructional cations, bentonite was a suitable additive for promoting AD performance |
[19] |
| Adding bentonite at organic loading rate of 1.39 g VS L−1 d−1. (VS, volatile solid) | Enhanced by 17.7% methane production | Bentonite contains many metal ions, providing necessary elements for the microorganism. Metal ions can be used as energy supplements to change the cell permeability, promoting microorganism to absorb nutrients selectively |
[20] |
Anaerobic fermentation to yield biogas is affected by various factors, such as composition, salinity, alkalinity, residence time, solid–liquid ratio, temperature, pH, C/N ratio, organic loading, bacterial vaccination degree, toxicity type, hydraulic residence time, agitation/mixture, pretreatment process, and reactor [21][22][23]. The research showed that kitchen rice, vegetables, meat, and a mixture of kitchen waste produced 478.2, 433.3, 206.8 and 508.3 mL/g biogas, respectively, indicating that mixing waste is favorable for biogas production [21][22][23]. Salinity affects the normal growth of methane bacteria, resulting in an increase in salinity outside the bacterial cell and the loss of water from the cells, thereby reducing the activity of microorganisms and even resulting in the complete failure of the fermentation system [24]. Two methods can be performed as countermeasures to protect the bacterial cell from losing water in the high salinity system. The first method is the synthesis and accumulation of osmotic protectants. The second method is adding an influx of potassium and chloride into the cytoplasm [24][25]. Fermentation may produce free ammonia, volatile aliphatic acid and sulfide/sulfate, which are important nutrients for bacterial growth; however, their increased concentrations will inhibit biogas formation [26]. For example, in the fermentation process, ammonia nitrogen mainly exists in two forms, namely, free ammonia and ammonium ions, which directly or indirectly inhibit the anaerobic fermentation process, especially free ammonia, which has stronger toxicity. Free ammonia can penetrate the cell membranes of bacteria, disturb the proton balance, change the pH value in cells, inhibit the activity of specific enzymes, and eventually lead to the deterioration of the anaerobic digestion process [27][28]. A submersible microbial desalination cell was developed to lower the ammonia level by in situ ammonia recovery and electricity generation, with a significant increase in biogas production. By gradually increasing the ammonia nitrogen concentration, the bacteria could gradually adapt to the high ammonia concentration, which could also reduce the inhibition effect of ammonia on AD [27][28]. The research showed that mesophilic bacteria could be adapted to a total ammonia nitrogen (TAN) concentration up to 5 g/L by means of gradual TAN loading. The cumulative biogas production in reactors with gradual TAN loading (for maximum TAN of 10 g/L) was 1.9–3.1 times more than that with abrupt TAN addition [27][28].
In addition to the above factors, the addition of a third component, such as biocarbon or a trace element, can improve the biogas yield of kitchen waste. The gas production effect of the anaerobic digestion of food waste was the best, and the methane yield was 331.7 L/kg TS. When the inoculation amount of sludge was 20.98%, the initial pH was 7.05, and with the addition of 22.14 g/L biocarbon, the maximum kitchen waste anaerobic digestion was realized, namely, 331.7 L/kg TS [29]; however, the mechanisms of biocarbon synergism in anaerobic fermentation are still under discussion. For example, one mechanism is put forward that biocarbon can promote electron transfer between species, enhance cell colonization and increase enzyme activity to promote biogas production [20][21]; another proposed mechanism suggests that the addition of biocarbon can improve the availability of the trace elements and beneficial elements that are necessary for methane production, and can reduce the loss of nutrients during fermentation [30].
Proper supplementation with microelements can improve methane yield [31]. In semicontinuous acclimatization, the daily addition of Fe(0) species to kitchen waste in sludge for anaerobic fermentation resulted in a 17.74% higher average daily biogas production than the control group; i.e., Fe(0) was found to be more conducive to improving the stability and yield of biogas for the single-phase anaerobic digestion of kitchen waste [32]. The combination of FeCl2 and EDTA was shown to promote protein polysaccharide hydrolysis and improve the degradation of volatile acids in the 13 d reaction. The yield of gas was 4.1 times that of when only FeCl2 was added [33].
2. Biohythane
Biohythane is a hydrogen–methane blend with a hydrogen concentration of 10 to 30% v/v [34]. Biohythane is derived from organic degradable products in a secondary anaerobic digestion process. In two-stage anaerobic fermentation, hydrogen microbes play an important role in the short residence time in the first stage, even though they produce methane for a relatively long time. Compared with traditional fuels, biohythane is valuable on the market and is universally accepted as a commercial fuel for use in internal engines and automobiles [35]. Environmentally, biohythane is advantageous for decreasing greenhouse gases because the presence of hydrogen decreases the carbon content [36].
Biohythane is produced in a dark fermentation stage and an acetyl-hydrogen stage, and each stage is controlled by microbes. Each stage has its own optimal conditions, such as pH value, fermentation temperature, partial pressure of methane, hydraulic residence time, organic load and nutrient composition [34].
Compared with the one-stage system, the two-stage biohythane system achieved a higher COD removal rate and energy recovery rate. The first stage of hydrogen fermentation benefited subsequent biomethane production [36]. Furthermore, two-stage anaerobic methods are more commercially valuable than one-stage methods; this is because dark fermentation results in both the poor yield and productivity of hydrogen, and is economically unsustainable [37].
However, because of its high required investment and operation complexity, the applicability of the two-stage method is severely limited. Recent research on the anaerobic one-stage production of biohythane is confined to the use of one digestion pool in order to simultaneously produce hydrogen and methane [38][39]. The reactor design, reactor operation strategy, and collection mechanism of biohydrogen in the separation pattern are unknown [40].
Chen’s research on the two-phase fermentation of kitchen waste in order to produce biohythane reveals that a low ammonia nitrogen concentration has a positive effect on hydrogen formation. However, a high concentration has a negative effect or even causes complete interruption. A 9.4 g VS /L.d organic load, 4 d hydraulic residence time, 1.0 reflux ratio for the hydrogen-producing phase, and 20 d hydraulic residence time for the alkane-producing phase are favorable conditions for the capture of biohythane, with a 14.2% H2/CH4 ratio [41].
The accumulation yield of biohythane varies with the organic load (OL, Table 2). At various OL values, the methane fraction increased with time, contributing to the presence of hydrogen and volatile aliphatic (fatty) acids (VFAs) at the top of the reactor. VFAs are good precursor substrates for methane because the residue of acid-producing bacteria is the substrate of methane-producing bacteria. Several steps were used to induce the formation of methane, with a maximum methane content of 35% at an OL of 100 g/L COD. Increasing the organic load produced a considerable increase in VFA production. The improved yield in the fatty acid can be attributed to the resulting suppression of methanogenic activity during the feeding stage [42].
Table 2. Cumulative biohythane and VFA production recorded at different OLs.
| OL (g COD/L) | 60 (Control) | 60 | 70 | 80 | 90 | 100 |
| Biohythane (L) | 128.7 | 144 | 156 | 156 | 159 | 163 |
| VFA (mg/L, 36 h of the cycle operation) | 4596 | 5087 | 5869 | 6155 | 6466 | 6754 |
3. Biohydrogen
The combustion of hydrogen in air produces water with a high LHV (lower heating value, 120 MJ/kg) that is 2.5, 2.3, 2.6 and 2.4 times the LHV of the combustion with the same amount of CNG (compressed natural gas), LNG (liquefied natural gas) and LPG (propane and methane), respectively (Table 3). Hydrogen is considered to be the most extractable clean and renewable energy source [43]. The fermentation of anaerobic organic microbes is a key pathway for biological hydrogen production. There is a considerable amount of organic garbage in agricultural residue, kitchen waste and industrial wastewater.
| Fuel | CNG | LNG | LPG | H2 | CH4 |
|---|---|---|---|---|---|
| LHV (MJ/kg) | 47.45 | 51.85 | 46.30 | 120.0 | 50.2 |
| Research octane number | >127 | >127 | 109 | Not established | 107 |
| Motor octane number | 122 | 122 | 96 | Not established | - |
| Boiling point (°C) | — | −162 | −42.09 | −252.8 | −161.5 |
| Explosion limits (% vol) | 5–15 | 5–15 | 2.1–9.5 | 4.0–74.2 | 5.0–15.4 |
| Self-ignition temperature (°C) | 650 | 650 | 450 | 580 | 538 |
(1) Octane number is a measure of the resistance of fuels to detonation or ignition. Research octane number (RON) is measured with an engine speed of 600 rpm, while Motor octane number (MON) is measured with an engine speed of 900 rpm. (2) LHV is the net amount of heat produced by the combustion of a unit quantity of a fuel.
The microbial fermentation of organic matter under anaerobic conditions is an important method for biohydrogen production. Agricultural residues, food waste, industrial wastewater, vegetable wastes and other wastes containing high organic matter are economic raw materials for biohydrogen production. The combination of hydrogen production and pollutant removal is a promising method for energy recovery from wastes [2].
Many studies have investigated the use of kitchen waste as a substrate in order to produce biohydrogen, but this method is limited by various obstacles, such as the composition of the waste, the type of microbes, and the processing environment (pH, atmospheric pressure, temperature, hydraulic residence time, feeding rate, operating conditions, etc.). Reasonable pretreatment in hydrogen preparation is necessary in order for complex organics to improve the production performance. Biohydrogen is produced by different approaches, namely, biophotonolysis, electrolysis by microbes and fermentation (dark fermentation, dry fermentation, and photofermentation).
Dark fermentation has the suitable characteristics of low energy demand, high yield and productivity, in order to be considered for large-scale hydrogen production. In dark fermentation, in which a single bacterium or community is available, the microbe variety is critical to hydrogen production. Commonly used bacteria include Clostridium, Enterobacteriaceae, Bacteroides and Thermoanaerobium, among others [47].
In photofermentation, which is dependent on rays, organic substrates are converted into hydrogen by photosynthetic bacteria [48]. Purple sulfur bacteria, purple non-sulfur bacteria and green sulfur bacteria are photosynthetic bacteria that use light and organic waste to produce hydrogen. The advantages of photofermentation include its good hydrogen yield and low COD level due to its utilization of dark fermentation products [49]. However, this technique has various shortcomings, such as poor photoefficiency, the requirement of transparent reactors and high energy consumption [19].
Dark fermentation produces hydrogen at a high rate, while photofermentation produces hydrogen at a good yield. Therefore, a suitable combination of the two could yield the maximum hydrogen output, which should be further investigated in the future [50]. For example, the researchers used the respective advantages of dark fermentation and light fermentation to implement the two-step coupling of dark–light fermentation, with kitchen waste as the substrate. By optimizing the H2 production conditions of dark fermentation and light fermentation, the following results were obtained: (1) at 37 °C, a 50% feed ratio, and an initial pH = 6, dark fermentation produced 25.18 mL/g of VS hydrogen, and (2) at 30 °C, 100 W illumination, an initial pH = 8, and 10% vaccination, photofermentation produced 34.62 mL/g of VS hydrogen. The total for the two fermentation methods produced up to 59.80 mL/g of VS hydrogen, significantly increasing the hydrogen yield of kitchen waste [51].
Anaerobic fermentation to generate hydrogen is classified into wet fermentation (less than 15% solid content) and dry fermentation (greater than 15% solid content). Compared with wet anaerobic fermentation, dry anaerobic fermentation has many advantages, such as lower water consumption, higher gas production, reduced biogas slurry, and the lower moisture content of biogas residues [40]. Synergistic dry fermentation with different substrates could improve hydrogen production efficiency. For example, when the ratio of kitchen waste to garden waste was 60:40, the maximum cumulative hydrogen production reached 85.28 NmL/g VS by dry fermentation at 55 °C, which was 1.35 times higher than that obtained using single kitchen waste and 1.93 times that obtained using a single garden waste [40].
Despite these results, industrial-scale anaerobic fermentation to produce biohydrogen has not been adopted due to technical obstacles, its high cost and poor efficiency. Improving the hydrogen yield is a continuous concern in academia. New ideas, such as substrate addition, the continuous removal of products, and removal inhibition caused by substrate and product accumulation, have been proposed [52]. Genetic engineering to modify bacterial strains to reduce side products [53] and the use of nanoparticle additives to enhance enzymatic activity [54] have also been proposed.
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9030247
References
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manag. 2019, 241, 619–630.
- Bhatia, R.K.; Ramadoss, G.; Jain, A.K.; Dhiman, R.K.; Bhatia, S.K.; Bhatt, A.K. Conversion of Waste Biomass into Gaseous Fuel: Present Status and Challenges in India. BioEnergy Res. 2020, 13, 1046–1068.
- Gao, Y. Study on the Domestication of Propionic Acid Methanogens and the Enhancement of Anaerobic Fermentation of Food Waste; Northeast Agricultural University: Harbin, China, 2018.
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on microbial community in anaerobic digestion with emphasis on environmental parameters: A systematic review. Chemosphere 2021, 270, 128618.
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217.
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195.
- Ma, Y.; Gu, J.; Liu, Y. Evaluation of anaerobic digestion of food waste and waste activated sludge: Soluble COD versus its chemical composition. Sci. Total Environ. 2018, 643, 21–27.
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. 2019, 37, 107414.
- Panepinto, D.; Genon, G. Analysis of the extrusion as a pretreatment for the anaerobic digestion process. Ind. Crops Prod. 2016, 83, 206–212.
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12.
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149.
- Ariunbaatar, J.; Panico, A.; Yeh, D.H.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Enhanced mesophilic anaerobic digestion of food waste by thermal pretreatment: Substrate versus digestate heating. Waste Manag. 2015, 46, 176–181.
- Li, Y.; Jin, Y. Effects of thermal pretreatment on acidification phase during two-phase batch anaerobic digestion of kitchen waste. Renew. Energy 2015, 77, 550–557.
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599.
- Zhu, H. Study on Anaerobic Digestion of Food Waste: Effect of Pretreatment; Zhejiang University: Hangzhou, China, 2018.
- Feng, L.; LI, R. Efficiency of anaerobic digestion of kitchen waste by low intensity ultrasound pretreatment. Chin. J. Environ. Eng. 2012, 6, 3280–3286.
- Sun, Y. Effect of Different Pretretments on Fermentative Hydrogen and Methane Production from Food Waste and Mechanism Investigation; Beijing University of Chemical Technology: Beijing, China, 2013.
- Linyi, C.; Yujie, Q.; Buqing, C.; Chenglong, W.; Shaohong, Z.; Renglu, C.; Shaohua, Y.; Lan, Y.; Zhiju, L. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ. Res. 2020, 188, 109743.
- Wang, P.; Wang, X.; Chen, X.; Ren, L. Effects of bentonite on antibiotic resistance genes in biogas slurry and residue from thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2021, 336, 125322.
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon-based functional materials. Bioresour. Technol. 2018, 266, 555–567.
- Zhang, D.; Duan, N.; Tian, H.; Lin, C.; Zhang, Y.; Liu, Z. Comparing two enhancing methods for improving kitchen waste anaerobic digestion: Bentonite addition and autoclaved de-oiling pretreatment. Process Saf. Environ. Prot. 2018, 115, 116–124.
- Yin, X. Optimization of Anaerobic Fermentation Process with Mixed Materials of Kitchen Waste and Vetiveria Zizanioides; Nanyang Normal University: Nanyang, China, 2018.
- Yi, L.; Rao, L.; Wang, X.; Wang, H. Effect of ventilation on nitrogen conversion and nitrogen loss in kitchen waste compost. J. Cent. South Univ. 2012, 43, 1584–1588.
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. AMB Express 2018, 8, 1.
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2.
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438.
- Akindele, A.A.; Sartaj, M. The toxicity effects of ammonia on anaerobic digestion of organic fraction of municipal solid waste. Waste Manag. 2018, 71, 757–766.
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239.
- Ma, S. Study on Biochar Promoting Anaerobic Digestion of Gas Production from Kitchen Waste; Huazhong University of Science and Technology: Wuhan, China, 2018.
- Qi, Q.; Sun, C.; Zhang, J.; He, Y.; Wah Tong, Y. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chem. Eng. J. 2021, 406, 126833.
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379.
- Wei, T.; Wen, H.; Chen, J. The effect of zero-valent iron acclimated sludge on methane production from kitchen wastes by anaerobic digestion. Hubei Agr. Sci. 2016, 55, 3618–3621.
- Liu, Y.; Yu, Z.; Meng, X.; Ding, q.; Zhang, H. Effects of Fe2+ dosing methods on anaerobic digestion of kitchen waste. Appl. Chem. Ind. 2019, 48, 838–840.
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent developments in biohythane production from household food wastes: A review. Bioresour. Technol. 2018, 257, 311–319.
- David, B.; Federico, B.; Cristina, C.; Marco, G.; Federico, M.; Paolo, P. Chapter 13—Biohythane Production from Food Wastes. In Biohydrogen, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–368.
- Si, B.; Liu, Z.; Zhang, Y.; Li, J.; Shen, R.; Zhu, Z.; Xing, X. Towards biohythane production from biomass: Influence of operational stage on anaerobic fermentation and microbial community. Int. J. Hydrogrn Energy 2016, 41, 4429–4438.
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Pereira, M.A. Boosting dark fermentation with co-cultures of extreme thermophiles for biohythane production from garden waste. Bioresour. Technol. 2016, 219, 132–138.
- Pasupuleti, S.B.; Venkata Mohan, S. Single-stage fermentation process for high-value biohythane production with the treatment of distillery spent-wash. Bioresour. Technol. 2015, 189, 177–185.
- Vo, T.-P.; Lay, C.-H.; Lin, C.-Y. Effects of hydraulic retention time on biohythane production via single-stage anaerobic fermentation in a two-compartment bioreactor. Bioresour. Technol. 2019, 292, 121869.
- Ta, D.T.; Lin, C.-Y.; Ta, T.M.N.; Chu, C.-Y. Biohythane production via single-stage anaerobic fermentation using entrapped hydrogenic and methanogenic bacteria. Bioresour. Technol. 2020, 300, 122702.
- Chen, X. Ammonia Nitrogen Characteristics and Control Strategies for Two-Phase Anaerobic Digestion of Food Waste; Zhejiang University: Hangzhou, China, 2014.
- Sarkar, O.; Venkata Mohan, S. Pre-aeration of food waste to augment acidogenic process at higher organic load: Valorizing biohydrogen, volatile fatty acids and biohythane. Bioresour. Technol. 2017, 242, 68–76.
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresour. Technol. 2018, 267, 71–76.
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504.
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898.
- The Engineering ToolBox. Fuels-Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com (accessed on 1 February 2023).
- Liu, X.; Bao, Z.; Pen, J.; Yang, L.; Shi, Y.; Liu, H. Review of biohydrogen production by anaerobic fermentation of food waste. J. Tianjin Agric. Univ. 2017, 24, 95–99.
- Hoàng, T.Y.; Khoo, K.S.; Ngọc, H.L.T.; Thu, Q.T.T.; Thị, T.Đ.; Thu, H.Đ.T.; Hoàng, H.C.; Chinthalapati, S.; Lay, C.-H.; Show, P.L. Sustainable cultivation via waste soybean extract for higher vaccenic acid production by purple non-sulfur bacteria. Clean Technol. Environ. Policy 2021, 23, 103–112.
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogrn Energy, 2022; in press.
- Saravanan, A.; Senthil Kumar, P.; Khoo, K.S.; Show, P.-L.; Femina Carolin, C.; Fetcia Jackulin, C.; Jeevanantham, S.; Karishma, S.; Show, K.-Y.; Lee, D.-J.; et al. Biohydrogen from organic wastes as a clean and environment-friendly energy source: Production pathways, feedstock types, and future prospects. Bioresour. Technol. 2021, 342, 126021.
- Zeng, X. tudies on hydrogen production from kitchen waste by sequential dark-and photo-fermentation. Environ. Sci. Manag. 2013, 38, 152–155.
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458.
- Mishra, P.; Wahid, Z.A.; Karim, A.; Pant, K.K.; Ghosh, P.; Kumar, D.; Singh, L. Chronological perspective on fermentative-hydrogen from hypothesis in early nineteenth century to recent developments: A review. Biomass Convers. Biorefin. 2022, 12, 3711–3723.
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int. J. Hydrogrn Energy 2019, 44, 13106–13113.
This entry is offline, you can click here to edit this entry!
