The current review comprehensively covers major research to evaluate the effectiveness of PGPR in alleviating crop water stress and to find effective PGPR to help crops in maintaining water status under drought conditions. The aim of the present review is to provide insights into the role of phytohormones, plant metabolites, exopolysaccharides (EPS), and 1-aminocyclopropane-1-caroboylic acid (ACC) deaminase activity in stress tolerance of plants in response to PGPR inoculation. This review identifies the challenges of drought stress and involvement of PGPR in the mitigation of drought stress in plants for sustainable production.
- Rhizobacteria
- Water Conservation
- Arid Lands
1. Introduction
Desertification, drought, and land degradation are major challenges to sustainable crop production throughout the world especially in developed countries. Water scarcity mainly due to low annual precipitation is very damaging for plant growth, and ultimately sustainable crop production. However, there is an inordinate need to use these areas even with marginal productivity due to damage to basic farmlands. Therefore, there is more interest in producing crops using low or marginal yields of soil (e.g., sandy soil) [1]. However, sandy soil has high temperatures and suffers severe drought. Stress losses can range from 50% to 80%, depending on the stress period and type of plant species [2]. Drought stress in desert areas affects plant water potential, restricts the normal plant performance,[3], and alters the plant physiological and morphological characteristics [4][5]. Drought stress-induced plant growth was studied in wheat [6], barley [7], rice, and corn[8]. Moisture content and plant biomass are common growth factors impacted by drought stress in these plants [9]. Besides, drought stress stimulus negatively impacts the nutrient uptake and translocation as the soil nutrients are transferred to the roots via water.
Consequently, drought stress reduces the absorption of nutrient and mass-flux of water-soluble nutrients, for example, calcium, nitrate, sulfate, silicon, and magnesium[10]. Drought stress enhances formation of free radicals that damage plant defence system resulting in an increase in reactive oxygen species (ROS), such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide induces oxidative stress. ROS can cause tissue damage, to membrane corrosion, proteins and nucleic acids by causing their lipid peroxidation [11][12][13].
Water stress is responsible for high economic losses in arid and semi-arid regions. It disturbs plant–water relations at cellular and whole plant levels, resulting in specific and non-specific responses [14]. Plant reaction to water stress is a complex process that tends to include polyamine formation and a collection of novel proteins with relatively unknown functions. Drought decreases the photosynthesis supply of carbon dioxide, which may contribute to ROS production from misguided electrons in the camera system[15][16]. It also creates free radicals during abiotic tension. ROS, such as radical superoxide (O2−), radical hydroxyl (OH), and hydrogen peroxide, enhance the damaging effect of lipid peroxidation throughout the membrane[17]. Plants have an antioxidant defensive system which prevents cellular membranes and DNA from ROS-induced oxidative damage by converting ROS into non-toxic forms such as water and oxygen[18][19].
Inoculation of plants with growth-promoting microorganisms can improve water retention strategies and drought tolerance of plants grown in arid or semi-arid regions [20]. These useful microbes inhabit the rhizosphere/endogenous rhizosphere of the plant through various direct-indirect mechanisms and promote plant growth (Figure 1). The rhizosphere is a thin layer of soil surrounding the roots of the plant and is a very critical and active area of root activity and metabolism[21][22][23]. A significant number of microorganisms coexist in the rhizosphere, such as bacteria, fungi, protozoa, and algae, but mostly different types of bacteria. Plants release organic compounds through exudate to select the bacteria that contribute most to the plant’s health under stressful conditions [24]. The beneficial relationships of plant-microbes in the rhizosphere are the key determinants in water conservation, soil productivity, and plant health. Plant growth-promoting rhizobacteria (PGPR) affect growth, yield, and nutrient uptake through a series of mechanisms. Some strains (e.g., Azospirillum brasilense, Aeromonas punctata, Bacillus megaterium, Pseudomonas fluorescens, Serratia marcescens) directly modulate plant physiology by stimulating the production of plant hormones, while others upturn minerals and nitrogen in the soil as a means of increasing growth under water-deficient conditions[25][26][27][28].
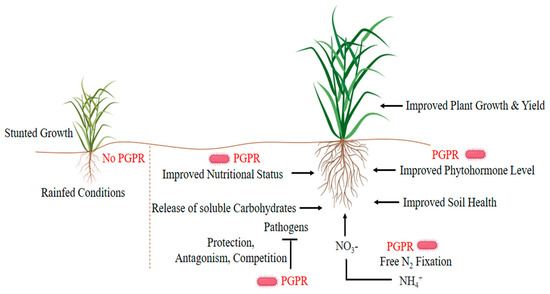
Figure 1. Plant growth-promoting strategies of plant growth-promoting rhizobacteria (PGPR) under drought stress.
2. Plant Survival Strategies under Drought Stress
A species may have a complementary set of survival strategies enabling it to survive under small and unpredictable distribution of rain[29]. Desert plants may have no water for many years. Plants exhibit different responses when sensing abiotic stimuli, which are related to specific stress-tolerance mechanisms[30][31][32]. A series of epidermis waxes protect plants from excessive moisture loss and provide protection against various pathogenic antagonistic activities [33]. In addition, osmoprotectants like proline accumulation aid in sustaining the plant’s water potential, and promotes the plant’s extraction of water from the soil[34]. Changes in primary metabolism are considered to be the most obvious of all metabolic reactions and comprise changes in the level of sugar/sugar alcohol, amino acid, and tricarboxylic acid cycle intermediates, exhibiting a common tendency for ecological stress reactions. However, changes in secondary metabolism are exact to particular stress and are precise to the type of plant species[35][36].
Some of the metabolic compounds that are associated with abiotic stresses and act as protectants include the sorbitol, polyols, mannitol, sucrose, fructan, proline, and ectoine[37]. Other small molecules such as carotenoids, ascorbic acid, tocopherols and anthocyanins also protect plants from being subjected to oxidative injury and protect plants by eliminating stress-induced ROS in plants. The production of phytoalexins and initiation of phenylpropanoid pathways and lignin biosynthesis are related to plant defence mechanisms[38][39][40]. Plant molecules such as salicylic acid, jasmonic acid, methyl salicylate, and methyl jasmonate are formed under stress. They can also act as signalling molecules that trigger defences against various biotic and abiotic stresses in crop plants [41]. In recent years, metabolomics has been used for various purposes, such as (1) assessing the effect of various stresses in plants; (2) pursuing the contribution of specific compounds in a specific biosynthetic or secondary deprivation pathway and (3) organizing various plant samples[42]. Stability, defence, and signalling of metabolites can be used to measure the degree of plant lenience to diverse abiotic stresses [43][44]. Extensive research is carried out to develop policies against drought stress by growing drought-tolerant crops, improving crop calendars and resource management practices [45].
This entry is adapted from the peer-reviewed paper 10.3390/agronomy10111683
References
- Campbell, A. The use of wild food plants, and drought in Botswana. J. Arid. Environ. 1986, 11, 81–91.
- Christensen, J.H.; Christensen, O.B. A summary of the PRUDENCE model projections of changes in European climate by the end of this century. Clim. Chang. 2007, 81, 7–30.
- Hsiao, T.C.; Xu, L. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616.
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586.
- Zhao, J.; Ren, W.; Zhi, D.; Wang, L.; Xia, G. Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress. Plant Cell Rep. 2007, 26, 1521–1528.
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152.
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremediat. 2018, 16, 405–414.
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.-K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2006, 58, 169–175.
- Kamara, A.Y.; Ekeleme, F.; Chikoye, D.; Omoigui, L.O. Planting Date and Cultivar Effects on Grain Yield in Dryland Corn Production. Agron. J. 2009, 101, 91–98.
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of Salinity Stress on Some Growth Parameters of Wheat by Exopolysaccharide-Producing Bacteria. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2725–2733.
- Dimkpa, C.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium byStreptomyces tendaeF4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696.
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought Stress in Wheat during Flowering and Grain-filling Periods. Crit. Rev. Plant Sci. 2014, 33, 331–349.
- Nair, P.R. Agroecosystem management in the 21st century: It is time for a paradigm shift. J. Trop. Agric. 2008, 46, 1–12.
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; Von Korff, M.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J. Exp. Bot. 2009, 60, 3531–3544.
- Venuprasad, R.; Lafitte, H.R.; Atlin, G.N. Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crop. Sci. 2007, 47, 285–293.
- Gaffney, J.; Schussler, J.; Löffler, C.; Cai, W.; Paszkiewicz, S.; Messina, C.D.; Groeteke, J.; Keaschall, J.; Cooper, M. Industry-Scale Evaluation of Maize Hybrids Selected for Increased Yield in Drought-Stress Conditions of the US Corn Belt. Crop. Sci. 2015, 55, 1608–1618.
- Akram, R.; Natasha; Fahad, S.; Hashmi, M.Z.; Wahid, A.; Adnan, M.; Mubeen, M.; Khan, N.; Rehmani, M.I.A.; Awais, M.; et al. Trends of electronic waste pollution and its impact on the global environment and ecosystem. Environ. Sci. Pollut. Res. 2019, 26, 16923–16938.
- Denef, K.; Roobroeck, D.; Wadu, M.C.M.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil. Biol. Biochem. 2009, 41, 144–153.
- Nasim, W.; Amin, A.; Fahad, S.; Awais, M.; Khan, N.; Mubeen, M.; Wahid, A.; Rehman, M.H.; Ihsan, M.Z.; Ahmad, S.; et al. Future risk assessment by estimating historical heat wave trends with projected heat accumulation using SimCLIM climate model in Pakistan. Atmos. Res. 2018, 205, 118–133.
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125.
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000.
- Yasmin, F.; Othman, R.; Sijam, K.; Saad, M.S. Effect of PGPR inoculation on growth and yield of sweetpotato. J. Biol. Sci. 2007, 7, 421–424.
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain P hyllobacterium brassicacearum STM 196 induces a reproductive delay and physiological changes that result in improved drought tolerance in A rabidopsis. New Phytol. 2013, 200, 558–569.
- Barnawal, D.; Singh, R.; Singh, R.P. Role of Plant Growth Promoting Rhizobacteria in Drought Tolerance. In PGPR Amelioration in Sustainable Agriculture; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 107–128.
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527.
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2015, 66, 35–42.
- Wang, C.; Guo, Y.; Wang, C.; Liu, H.; Niu, D.; Wang, Y.; Guo, J. Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR156. J. Agric. Biotechnol. 2012, 20, 1097–1105.
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514.
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164.
- Vaishnav, A.; Choudhary, D.K. Regulation of Drought-Responsive Gene Expression in Glycine max L. Merrill is Mediated Through Pseudomonas simiae Strain AU. J. Plant Growth Regul. 2018, 38, 333–342.
- Ansari, F.A.; Ahmad, I. Alleviating Drought Stress of Crops through PGPR: Mechanism and Application. In Microbial Interventions in Agriculture and Environment; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 341–358.
- Zheng, W.; Zeng, S.; Bais, H.; Lamanna, J.M.; Hussey, D.S.; Jacobson, D.L.; Jin, Y. Plant Growth-Promoting Rhizobacteria (PGPR) Reduce Evaporation and Increase Soil Water Retention. Water Resour. Res. 2018, 54, 3673–3687.
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101271.
- Armada, E.; Roldán, A.; Azcon, R. Differential Activity of Autochthonous Bacteria in Controlling Drought Stress in Native Lavandula and Salvia Plants Species Under Drought Conditions in Natural Arid Soil. Microb. Ecol. 2013, 67, 410–420.
- Venkateswarlu, B.; Shanker, A.K. Climate change and agriculture: Adaptation and mitigation stategies. Indian J. Agron. 2009, 54, 226–230.
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-arid Agroecosystem and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2018, 8, 2580.
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42.
- Piccoli, P.; Bottini, R. Abiotic Stress Tolerance Induced by Endophytic PGPR. In Soil Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; pp. 151–163.
- Panda, R.; Das, M.; Nayak, S. Estimation and optimization of exopolysaccharide production from rice rhizospheric soil and its interaction with soil carbon pools. Rhizosphere 2020, 14, 100206.
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martinez-Andujar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57.
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022.
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 1029.
- Jaleel, C.A.; Manivannan, P.A.R.A.M.A.S.I.V.A.M.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.A.M.A.M.U.R.T.H.Y.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105.
- Lucy, M. Management Strategies for Balance Herbicide in Chickpeas; GRDC: Canberra, Australia, 2004.
- Zhao, P.; Liu, P.; Shao, J.; Li, C.; Wang, B.; Guo, X.; Yan, B.; Xia, Y.; Peng, M. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: Ensuring survival or continuing growth. J. Exp. Bot. 2014, 66, 1477–1488.
