Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microcrystalline Cellulose (MCC) is an isolated, colloidal crystalline portion of cellulose fibers, and it is a valuable alternative to non-renewable fossil-based materials. It is used for a large plethora of different fields, such as composites, food applications, pharmaceutical and medical developments, and cosmetic and material industries. The interest of MCC has also been driven by its economic value. In the last decade, particular attention has been driven to the functionalization of its hydroxyl groups to expand the field of applications of such biopolymer.
- microcrystalline cellulose
- pre-treatments
- functionalization
- Applications
- organic derivatization
1. Introduction
In the last few years, the use of microcrystalline cellulose in functional components, such as MCC-fibres reinforced materials or exploiting its chemical functionality in order to modify the structure, has become an attractive field of research activity. The chemical derivatization of MCC with functional components leads to the formation of advanced materials with new or improved properties. Therefore, their functionalities will be improved, and potential applications in specific fields can be expanded. Functionalized MCC can be used as adsorbents (dyes, heavy metals, and carbon dioxide), flame retardants, reinforcing agents, energetic materials, and biomedical applications. Certainly, MMC, similar to all other cellulose derivatives, finds other useful applications in the field of cellulose-based lubricants, thanks above all to its outstanding dry-binding properties. However, due to the instability of the dispersion of chemical derivatization of MCC, they cannot be utilized directly as lubricants. Such dispersions can be made stable by adding a third component that can create a structural network to prevent aggregation and sedimentation of the MCC particles [1].
2. Adsorbents
Microcrystalline cellulose usually exhibits low adsorption ability toward dyestuffs. Therefore, its functionalization is necessary to obtain system with improved adsorption ability, as reported by Wei et al. They studied the functionalization of microcrystalline cellulose aerogel and its absorption efficiency towards dyes such as methylene blue. For this purpose, MCC was coated with polydopamine (PDA), which was realized via the self-polymerization of dopamine in the MCC/LiBr solution, followed by the freeze-drying step. The MCC/PDA system shows high adsorption efficiency and selectivity towards methylene blue from different solutions (mixed solution with common salts, cationic and anionic dyestuffs). Moreover, the high structural stability of the microcrystalline cellulose network makes the aerogel stable in aqueous conditions, and it has great potential application in wastewater treatment to reduce the emission of such contaminants, hence decrease water pollution [2].
Other systems based on MCC can be used as adsorbents of dyes. Hu et al. reported that microcrystalline cellulose functionalized with quaternary ammonium groups, using N,N-dimethyldodecylamine as nucleophile onto glycidyl moieties, is able to remove the Congo Red dye (CR) from aqueous solution. Ultrasound was used as a pre-treatment to disrupt the original structure of microcrystalline cellulose and make hydroxyl groups more accessible, as this is a two-step functionalization (Figure 1).
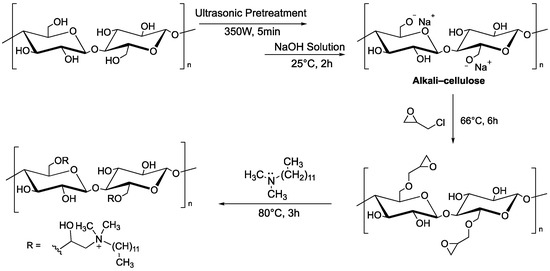
Figure 1. Two-step functionalization of MCC by reaction with glycidyl chloride, followed by ring opening with N,N-dimethyldodecylamine.
The adsorption of Congo Red dye depends on the solution’s pH, dye concentration, NaCl concentration, and temperature. The maximum adsorption capacity of MCC functionalized with N,N-dimethyldodecylamine reached 304.34 mg·g−1 (with a dye concentration of 80 mg·L−1, a volume of 100 mL, a temperature of 40 °C, and an adsorbent dosage of 10 mg) [3].
Tannic acid (TA) functionalized MCC was used for the selective recovery of gallium (Ga) and indium (In), as reported by Du et al. The functionalization was performed by radiation-induced grafting polymerization. The selective separation and recovery of Ga (III) and In (III) from potential leaching solutions were investigated using batch and fix-bed column experiments. Firstly, a radiation grafting technique prepared glycidyl methacrylate-grafted cellulose (MCC-g-GMA). The MCC-g-GMA was added to a TA aqueous solution to obtain the MCC-g-GMA-TA system (Figure 2). The functionalized MCC can recover Ga (III) from the simulated semiconductor waste-leaching solution containing As (III), Cu (II), Zn (II), and Ni (II) with a maximum adsorption capacity of 26.55 mg·g−1. On the other hand, In (III) can be separated from simulated Zn refinery residue leaching solution containing Cu (II), Zn (II), and Ni (II) with a maximum adsorption capacity of 35.63 mg·g−1. The FTIR, XPS analyses, and the ionic strength effect revealed that functionalized MCC can selectively recover gallium and indium by forming chelates between the hydroxyl groups and Ga+3 or In+2 [4].
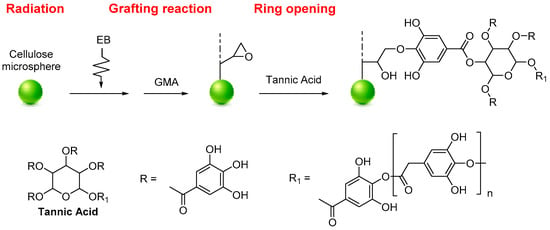
Figure 2. Synthesis of MCC-g-GMA-TA.
Microcrystalline cellulose can also be used to improve heavy metal adsorption from water and CO2 uptake. For this purpose, Rafieian et al. performed two successive grafting reactions for the chemical modification of MCC for the removal of heavy metals from industrial wastewater. The first reaction is the silylation by (3-chloropropyl)triethoxysilane (CPTES), and the second is the amine-functionalization with 1,1-dimethylbiguanide hydrochloride (Figure 3). The grafting of CPTES onto MCC surfaces involves the hydrolysis of the alkoxy groups of the silane and the formation of hydrogen bonds between the silanol obtained and the hydroxyl groups of cellulose with consequent adsorption and grafting onto -OH on the surface of MCC. Finally, the chemical condensation and the formation of a polysiloxane network on the surface of microcrystalline cellulose were performed [5].
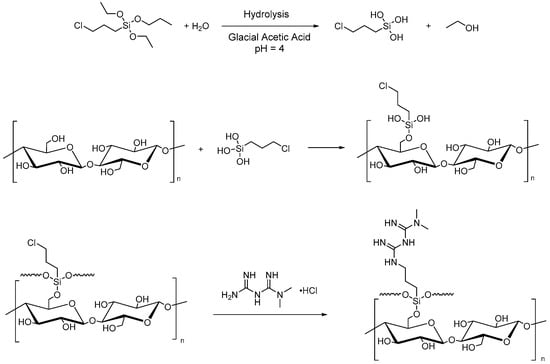
Figure 3. Silylation of MCC with CPTES and amine-functionalization (1,1-dimethylbiguanide hydrochloride).
On the other hand, Gunathilake et al. synthesized different cyanopropyl-incorporated microcrystalline cellulose-organosilica (MCC-CP) mesostructured materials in a two-step process for carbon dioxide sorption. They first performed a solvent evaporation-induced self-assembly of microcrystalline cellulose, tetraethyl orthosilicate, and (3-cyanopropyl)triethoxysilane in the presence of a triblock copolymer under acidic conditions. Cyanopropyl groups were further converted into amidoxime functionalities upon treatment with hydroxylamine hydrochloride (Figure 4). Different amidoxime-functionalized microcrystalline cellulose-mesoporous silica composites (MCC-AO) were obtained, and their CO2 sorption at ambient (25 °C) and elevated (120 °C) temperatures was evaluated. The composites showed low CO2 uptake at 25 °C, and sorption capacities between 2.15–3.85 mmol·g−1 are reached with high temperatures (120 °C). The values obtained confirm that MCC’s amine, oxime, and hydroxyl groups are active sites for CO2 sorption. Furthermore, the selectivity of these composites towards carbon dioxide in the presence of nitrogen, in addition to their low cost, high thermal and mechanical stability, non-toxicity, biodegradability, and biocompatibility, makes them potential candidates for CO2 sorption at elevated temperatures [6].
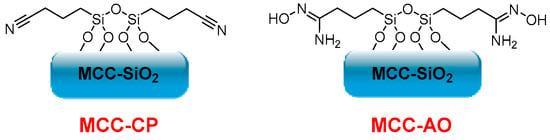
Figure 4. MCC-CP and MCC-AO structures.
3. Flame Retardants
Functionalized microcrystalline cellulose can be also used as a flame retardant. In particular, Yuan et al. reported the chemical modification of MCC with phytic acid (PA) by phosphorylation reaction for enhanced flame-retardant ability. The modified microcrystalline cellulose was synthesized at 90 °C with a 1:3 weight ratio of MCC to PA (Figure 5). The resulting system showed low heat release performance and good char-forming ability during thermal degradation. This work reveals that the PA phosphorylated MCC can act as a promising flame-retardant material [7].
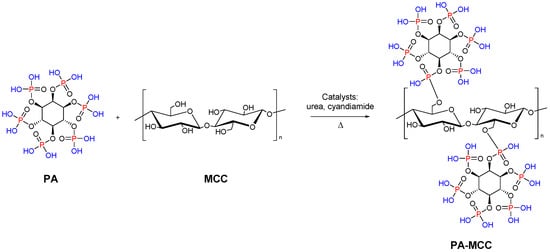
Figure 5. Synthesis of MCC modified with PA.
Finally, Lou et al. studied and synthesized a fully bio-based flame retardant with high efficiencies and mechanical reinforcement functions for epoxy resin. Microcrystalline cellulose was surface functionalized with chitosan (CS) and sodium phytate (PA-Na) via layer-by-layer assembly in water (Figure 6). The functionalized MCC catalyzes the degradation of epoxy resin to produce char residues with the formation of stable phosphate and nitride species in the condensed phase. The char can act as a physical barrier to inhibit smoke production and heat transfer. This result shows that the total heat release, total smoke production, peak heat release rate, fire growth rate, peak smoke production release, and fire retardancy index of the epoxy composites are greatly reduced, confirming their good fire-retardant ability. Moreover, adding 15 wt.% of modified microcrystalline cellulose can simultaneously enhance the composites’ strength, modulus, and toughness due to the satisfactory interfacial compatibility, favorable dispersion, and mechanical reinforcement effect of MCC [8].
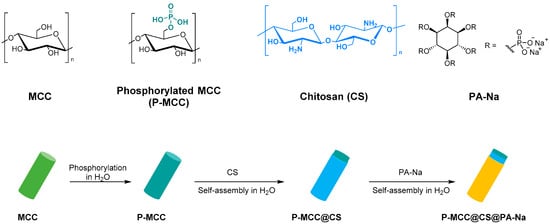
Figure 6. Synthesis P-MCC@CS@PA-Na.
4. Reinforcing Agent
Thanks to its renewability, biodegradability, low cost, and low density, MCC is considered a new class of cellulosic reinforcing agent that improves the sustainability of the final composite compared to silica, carbon black, and glass fibers.
Ashori et al. reported the use of microcrystalline cellulose as a reinforcing agent in polypropylene (PP)/microcrystalline cellulose (MCC)/wood flour composites containing polypropylene-graft-maleic anhydride (PP-g-MA) as a compatibilizer.
The weight ratio of the cellulosic materials to polymer matrix was 40:60 (w:w), and the final mechanical properties (flexural, tensile, and impact strengths) were significantly enhanced with the addition of microcrystalline cellulose, as compared with pure PP and composites without MCC. Scanning electron microscopy confirms that using maleic anhydride as a compatibilizer increases the fiber–matrix interaction. Moreover, the addition of PP-g-MA increases the degradation temperatures of composites with an improvement in thermal stability, which reaches the highest value when 5 wt.% of polypropylene-graft-maleic anhydride was used [9].
Ratnakumar et al. reported that microcrystalline cellulose acts as a reinforcement for polypropylene. They examined the properties of MCC-reinforced polypropylene composites varying the MCC loading from 0–5%. The final composite shows improved properties as the MCC loading increases. Tensile, hardness, and impact properties were improved by 5%, 8%, and 51% for 5 wt.% MCC-reinforced PP composite [10].
Microcrystalline cellulose was also used as a reinforcing agent in polydimethylsiloxane (PDMS)/MCC composites in order to improve the mechanical properties, as reported by Jankauskaite et al. Silver nanoparticles were synthesized in situ by chemical reduction method in MCC aqueous suspension to provide antimicrobial activity. Vinyl-terminated PDMS was mixed with MCC and silver nanoparticles, which combines stiffness and antimicrobial properties. However, voids around MCC demonstrate poor adhesion interaction at the PDMS matrix and MCC particle interface, as shown from SEM analysis in Figure 7.

Figure 7. SEM image of PDMS/MCC composites with 20 wt.% of filler tensile fractured surface at magnification of 2500× and 20 KeV.
Mechanical properties are improved compared to unfilled PDMS, and the final composite inhibited the growth and multiplication of the Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria after 1 h of incubation. However, the tendency of silver nanoparticles to aggregate and its low concentration led to bacteriostatic activity alone [11].
Deng et al. also reported the use of MCC as a reinforcing agent in silicones. Microcrystalline cellulose fibers from cotton linter were modified with propargyl bromide in an aqueous medium. The functionalized MCC was introduced as filler in silicone fluids, followed by crosslinking at high temperatures between hydride-functional and vinyl-functional PDMS to produce silicone elastomer composites. Furthermore, in the hydrosilylation process, it was expected to be also involved in the alkyne functionalized MCC (Figure 8). The presence of alkyne functions favors the interfacial adhesion between silicone and MCC and the densification of the network. The resulting composite showed an enhancement of mechanical and thermal properties when MCC-Alkyne was incorporated as filler, compared to unmodified MCC [12].
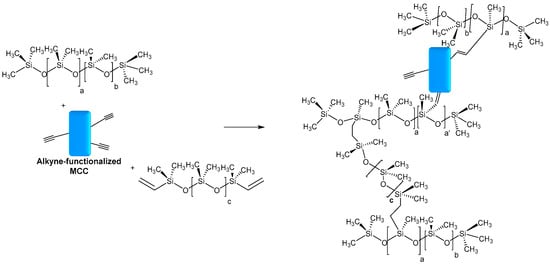
Figure 8. Possible hydrosilylation reaction occurring between alkyne-functionalized MCC and hydride-functional PDMS.
5. Energetic Materials
Among cellulose-based energetic materials, nitrocellulose is the most used in double-base rocket propellants and gunpowder. However, new alternatives have been investigated in the last years due to the high friability, low combustion temperature, chemical instability, and high sensitivity of nitrocellulose.
Tarchoun et al. reported the azide-modification of microcrystalline and pristine cellulose as promising nitrogen-rich energetic biopolymers. Azidodeoxy-microcrystalline cellulose nitrate (AMCCN) and azidodeoxypristine cellulose nitrate (APCN) were synthesized, and their properties were compared to cellulose nitrate (PCN) and microcrystalline cellulose nitrate (MCCN). PC and MCC were dissolved in DMAc/LiCl, and p-toluenesulfonyl chloride (hydroxyl activating agent), triethylamine (base catalyst), and subsequently sodium azide was added to obtain the corresponding azidodeoxy-pristine cellulose (APC) and azidodeoxy-microcrystalline cellulose (AMCC). Finally, the nitration of free-accessible hydroxyl groups of chemically functionalized and non-functionalized samples was performed with a mixture of fuming nitric acid and acetic anhydride to afford the corresponding PCN, MCCN, APCN, and AMCCN (Figure 9).
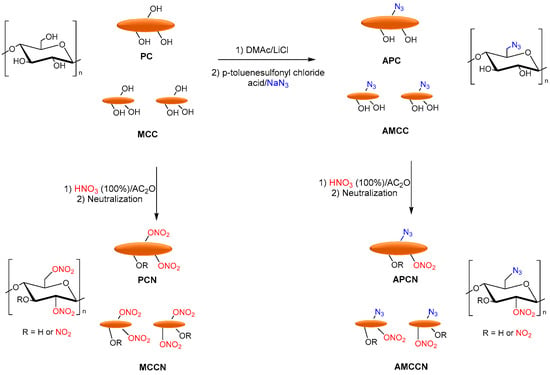
Figure 9. Synthesis of PCN, MCCN APCN, and AMCCN.
The newly synthesized AMCCN and APCN present high nitrogen contents, increased densities, and good thermal stabilities compared to the conventional PCN and microcrystalline cellulose nitrate. All nitrated polymers are insensitive against impact and friction, except for the nitrated unmodified ones (PCN and MCCN), which showed very high impact sensitivity. Furthermore, the experimentally measured heats of combustion and the predicted energetic performances of the investigated nitrated biopolymers indicated that the nitrated azide-modified biopolymers (APCN and AMCCN) displayed high heats of combustion, increased detonation velocities, comparable detonation pressures, low temperatures of explosion, and moderate specific impulses with respect to those of nitrated unmodified ones (PCN and MCCN) [13].
Tarchoun et al. also studied 6-deoxy-6-(ω-aminoethyl)nitramino microcrystalline cellulose nitrate (also referred to as microcrystalline cellulose amine nitrate, MCCAN) and 6-deoxy-6-(ω-aminoethyl)nitramino cellulose nitrate (also referred to as cellulose amine nitrate, CAN) as a new class of insensitive energetic nitrogen-rich polymers through chemical modification of cellulose and microcrystalline cellulose (MCC). Cellulose and MCC were dissolved in DMAc/LiCl, and p-toluenesulfonyl chloride with triethylamine was added to obtain cellulose tosylate (CT) and microcrystalline cellulose tosylate (MCCT). Then, the displacement of tosyl groups with ethylenediamine moieties was carried out in DMSO. Finally, 6-deoxy-6-(ω-aminoethyl)nitramino cellulose nitrate (CAN) and 6-deoxy-6-(ω-aminoethyl)nitramino microcrystalline cellulose nitrate (MCCAN) were synthesized with a mixture of fuming nitric acid and acetic anhydride (Figure 10).
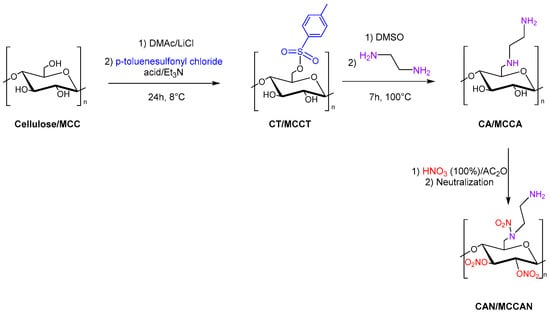
Figure 10. Synthesis of CAN and MCCAN.
The new synthesized energetic polymers (MCCAN, CAN) show good thermal stability, elevated densities, and high nitrogen content compared to cellulose nitrate and microcrystalline cellulose nitrate.
All nitrated polymers are insensitive to friction, whereas the aminated and nitrated polymers show extraordinarily lower impact sensitivities than those of unmodified polymers nitrate. The newly prepared CAN and MCCAN present high energy of the explosion, low detonation temperatures, comparable detonation pressures, high detonation velocities, and moderate specific impulses compared to the common cellulose nitrate and MCCN, respectively [14].
Finally, Tarchoun et al. reported the tetrazole-acetate modification of pristine cellulose (PC) and microcrystalline cellulose, using 1H-tetrazole-5-acetic acid as a functionalized agent. MCC and PC were dissolved in DMAc/LiCl, and p-toluenesulfonyl chloride with 1H-tetrazole-5-acetic was added to obtain 2-(1H-tetrazol-5-yl)acetate pristine cellulose (TAPC) and 2-(1H-tetrazol-5-yl)acetate microcrystalline cellulose (TAMCC). Finally, the nitration of OH groups of chemically modified and non-functionalized samples was carried out with a mixture of fuming nitric acid and acetic to afford the corresponding pristine cellulose nitrate (PCN), microcrystalline cellulose nitrate (MCCN), 2-(1H-tetrazol-5-yl)acetate pristine cellulose nitrate (TAPCN), and 2-(1H-tetrazol-5-yl)acetate microcrystalline cellulose nitrate (TAMCCN) (Figure 11).
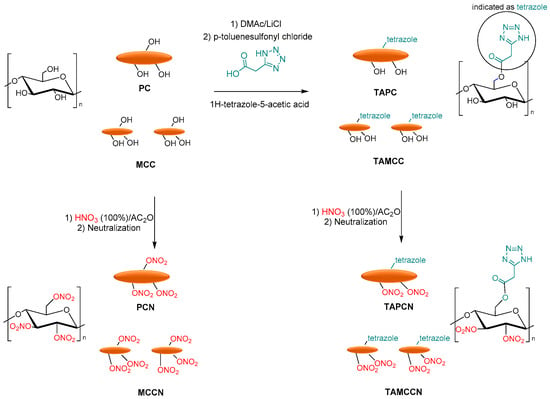
Figure 11. Synthesis of PCN, MCCN, TAPCN, and TAMCCN.
Additionally, in this case, the newly synthesized 2-(1H-tetrazol-5-yl) acetate pristine cellulose and microcrystalline cellulose showed high nitrogen contents, increased densities, and good thermal stability compared to the conventional pristine and MCC-based cellulose nitrates. The friction sensitivities of functionalized nitrated polymers were lower than those of non-functionalized nitrated polymers. Furthermore, the new tetrazole-acetate modified MCC and PC show low heats of the explosion, decreased detonation temperatures, similar detonation pressures, high detonation velocities, and moderate specific impulses compared to pristine cellulose nitrate and microcrystalline cellulose nitrate [15].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28052009
References
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrad, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment. A review. Int. J. Pharm. 2014, 473, 64–72.
- Huang, T.; Nie, J.; Yang, J.-H.; Qi, X.-D.; Zhou, Z.-W.; Wang, Y. Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohydr. Polym. 2018, 198, 546–555.
- Hu, D.; Wang, P.; Li, J.; Wang, L. Functionalization of Microcrystalline Cellulose with N,N-dimethyldodecylamine for the Removal of Congo Red Dye from an Aqueous Solution. Bioresources 2014, 9, 5951–5962.
- Du, J.; Zhang, M.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of tannic acid functionalized microcrystalline cellulose for selective recovery of Ga(III) and In(III) from potential leaching solution. Sep. Purif. Technol. 2022, 286, 120442.
- Rafieian, F.; Mousavi, M.; Yu, Q.; Jonoobi, M. Amine functionalization of microcrystalline cellulose assisted by (3-chloropropyl)triethoxysilane. Int. J. Biol. Macromol. 2019, 130, 280–287.
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-Functionalized Microcrystalline Cellulose-Mesoporous Silica Composites for Carbon Dioxide Sorption at Elevated Temperatures. J. Mater. Chem. A 2016, 4, 4808.
- Yuan, H.-B.; Tang, R.-C.; Yu, C.-B. Flame Retardant Functionalization of Microcrystalline Cellulose by Phosphorylation Reaction with Phytic Acid. Int. J. Mol. Sci. 2021, 22, 9631.
- Lou, G.; Ma, Z.; Dai, J.; Bai, Z.; Fu, S.; Huo, S.; Qian, L.; Song, P. Fully Biobased Surface-Functionalized Microcrystalline Cellulose via Green Self-Assembly toward Fire-Retardant, Strong, and Tough Epoxy Biocomposites. ACS Sustain. Chem. Eng. 2021, 9, 13595–13605.
- Ashori, A.; Nourbakhsh, A. Performance properties of microcrystalline cellulose as a reinforcing agent in wood plastic composites. Compos. B. Eng. 2010, 41, 578–581.
- Ratnakumar, A.; Rathnayake, W.S.M.; Karunanayake, L.; Samarasekara, A.M.P.B.; Amarasinghe, D.A.S. Microcrystalline Cellulose as Reinforcing Agents for Polypropylene Composites. Trop. Agric. Res. 2020, 31, 106–114.
- Jankauskaite, V.; Abzalbekuly, B.; Lisauskaite, A.; Procycevas, I.; Fataraite, E.; Vitkauskiene, A.; Janakhmetov, U. Silicone Rubber and Microcrystalline Cellulose Composites with Antimicrobial Properties. Mater. Sci. 2014, 20, 42–49.
- Deng, S.; Binauld, S.; Mangiante, G.; Frances, J.M.; Charlot, A.; Bernard, J.; Zhoub, X.; Fleurya, E. Microcrystalline cellulose as reinforcing agent in silicone elastomers. Carbohydr. Polym. 2016, 151, 899–906.
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Khimeche, K.; Mezrou, A. A promising energetic biopolymer based on azide-functionalized microcrystalline cellulose: Synthesis and characterization. Carbohydr. Polym. 2020, 249, 116820.
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B. New insensitive nitrogen-rich energetic polymers based on amino-functionalized cellulose and microcrystalline cellulose: Synthesis and characterization. Fuel 2020, 277, 118258.
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Khimeche, K. Tetrazole-functionalized microcrystalline cellulose: A promising biopolymer for advanced energetic materials. J. Chem. Eng. 2020, 400, 125960.
This entry is offline, you can click here to edit this entry!
