Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As the importance of farming continues to grow, innovative technology and sensors play an increasingly important role. Automation and robots in agriculture have the potential to play a significant role in helping society fulfill its future demands for food supply. Wearable sensors connected to or within cows can monitor eating, rumination, pH, body temperature, laying behavior, animal activity, animal position or placement, and more.
- biosensors
- precision dairy farming
- sensors technology
- dairy cattle
- early diagnosis
1. Introduction
Farm management is being significantly influenced by technological breakthroughs, which are reducing physical labor, expenditures, and waste while increasing yields and profits. As a result of agricultural technological breakthroughs, a new farming method called “precision agriculture” has evolved [1]. Monitoring real-time autonomic responses (e.g., respiration rate, heart rate variability and heart rate, blood pressure, changes in peripheral blood flow) and defense-related reflexes with innovative biosensor equipment can aid in understanding how housing, nutrition, and genotype influence animals’ resilience to stressors. These sensors can contribute to the knowledge of factors that influence animal welfare and the creation of remedies (for example, husbandry techniques, genotype selection) that increase the welfare of livestock and companion animals. Wearable sensors can track feeding behavior, rumination behavior, rumen pH, rumen temperature, body temperature, laying behavior, animal activity, and animal location or placement [2]. Wearable or imprinted biosensors that allow remote data transfer could be significant in this rapidly evolving field [3]. There are many gadgets that can measure body temperature, behavior, and movement of the animal [3][4][5][6]. Sensors and wearable technology can be inserted into animals to detect the components of their body fluids such as sweat [3][7][8]. Cows are also fitted with commercially available biosensor collars to monitor the estrous cycle [3][9]. Figure 1 demonstrates examples of various technologies applied to cows.
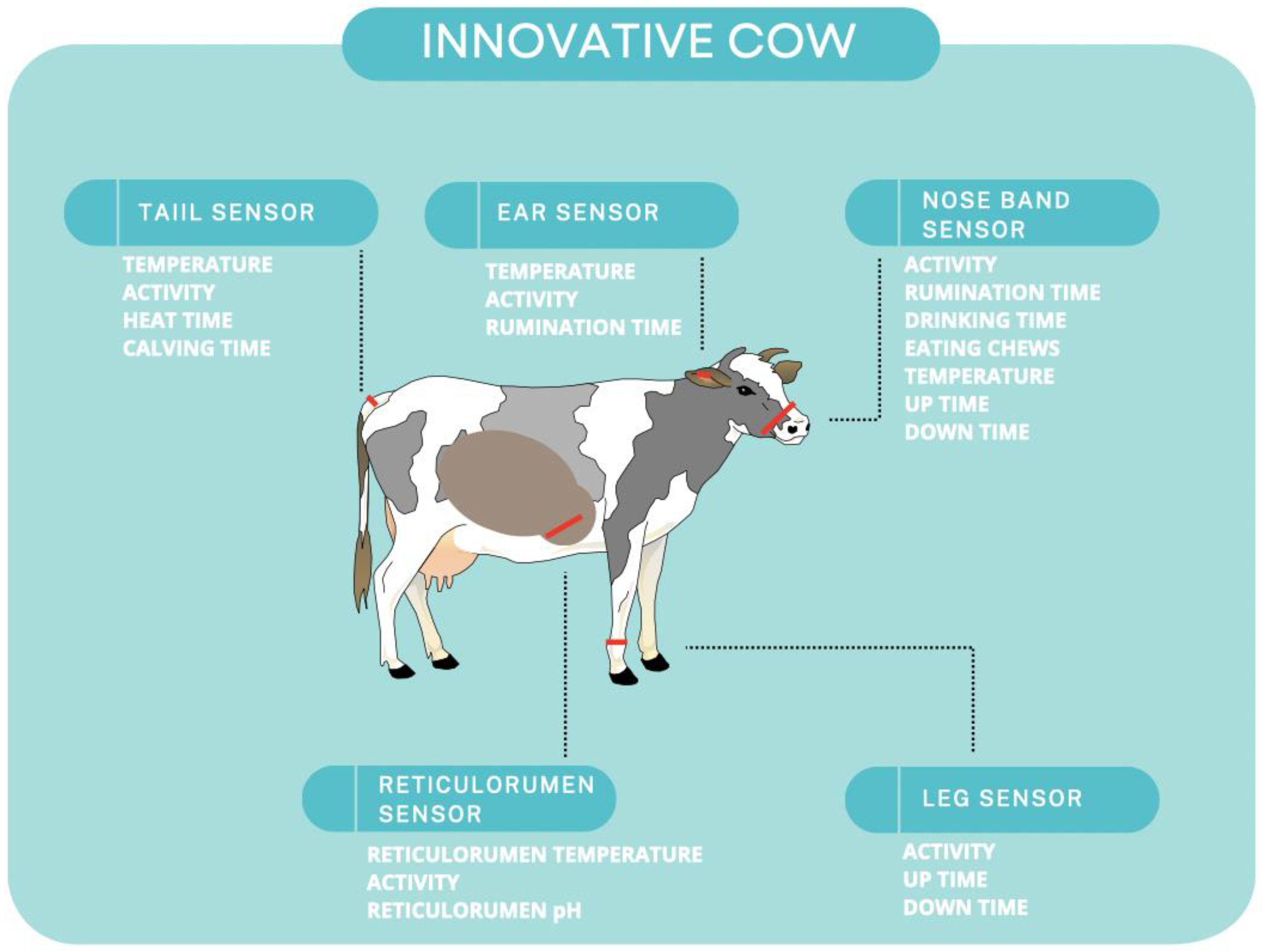
Figure 1. Innovative technologies applied to cows.
2. Milk Analyzers
The use of technologies to evaluate physiological, behavioral, and production markers on individual animals in order to identify events of interest is included in the process of precision dairy monitoring [10]. Milk analytes can be used as biomarkers for diseases or reproductive status detection. For example, DeLaval Herd Navigator (DeLaval Inc., Tumba, Sweden) detects the amount of progesterone in milk. The program also recommends the ideal timing for insemination, names animals that need to be confirmed as pregnant, flags early abortions, and lists cows at risk for cysts and protracted anestrus. Other milking robots such as Lely Astronaut A4 (Lely Campus, Cornelis van der Lely an 1, 3147, PB, Maassluis, The Netherlands) can determine milk analytes and milk electrical conductivity. Blood biomarkers are useful indicators of animal health, although they have limited economic use. They might provide a great deal of information, especially because biomarkers can detect subclinical stages of illnesses even when the cow appears fully healthy and shows no visible indications of illness. An alternative to blood biomarkers can be milk biomarkers [11]. Sensor devices for determining the fat and protein content of milk are widely utilized on farms nowadays. According to each farm’s milking system, a unique sensor system is utilized. These sensors provide information on the health and fertility of cattle. Using milk analyzers, the estrous cycle and reproductive performance in dairy cows can be tracked [3]. There are various analyzers which may operate on different principles, some using chemical indicators and others based on spectroscopy [12].
2.1. Somatic Cell Count
Mastitis-related milk production losses in dairy animals are economically significant [13]. Monitoring somatic cell count (SCC) concentrations in milk is the most widely used method for detecting mastitis, especially in its subclinical forms. When SCC values surpass the limit, the milk’s value plummets drastically. As a result, experts feel that SCC level is a significant parameter for evaluating udder health [14][15]. Despite the absence of clinical signs, subclinical mastitis is distinguished by an increase in SCC in milk. SCC is a useful udder health indicator since it counts the quantity of somatic cells (mainly desquamated epithelial cells, macrophages, and neutrophils) in milk. Aside from being used as a criterion for selecting dairy cows that are less susceptible to mastitis, the presence of SCC in bovine milk is a well-established indicator of mammary gland inflammation, which is strongly linked to the presence of a mammary infection. SCC has proven to be a useful indicator of decreased milk supply due to subclinical mastitis, which is present when the cell count is greater than 200,000 per milliliter [13][16][17][18][19].
Using cell staining methods and microscopy, the SCC concentration can be evaluated at the laboratory level. However, these approaches are time-consuming and call for specialized equipment and personnel [19]. Modern techniques for SCC in milk detection are much less laborious and time-consuming [19].
Automatic mastitis detection devices are examples of novel diagnostic processes that are field-adaptable, simple, and can rapidly provide results. There are many types of equipment such as the milk checker, Fossomatic meter (Hillerød, Denmark), Dramiski mastitis detector/Wykrywacz mastitis detector (Olsztyn, Poland), DeLaval cell counter (Tumba, Sweden), Afimilk mastitis detector (Kibbutz Afikim, Izrael), UdderCheck® test (Moorestown, USA), and PortaSCC® test (Moorestown, USA). They rely on either detecting physicochemical–biological alterations in milk or the udder or assessing biomarkers in body fluids (milk, serum) linked with mastitis [20].
It has been demonstrated that the diagnostic capacity of infrared thermography (IRT) is comparable to that of the California mastitis test, and it also distinguishes instances of clinical mastitis from those of subclinical mastitis. As a result, IRT has the potential to develop into a diagnostic tool that is both convenient and portable [20].
2.2. Milk Progesterone
Fertility control needs close coordination between farmers and veterinarians, systematic examination of farm records, and reliable clinical data. Furthermore, low fertility might be considered a sign of poor health and well-being [21]. Breeding is an essential element of cattle husbandry. Detecting the ovulation phase in cattle is crucial for determining the best window for artificial insemination [22]. The presence of progesterone implies the existence of a functioning corpus luteum. As a result, it has been utilized for decades as a biomarker of reproductive efficiency. Milk progesterone is a potential non-invasive indicator for reproductive status in dairy cows since progesterone is transported from blood to milk [21]. Therefore, progesterone sensors could be useful sensor systems, despite the fact that little research has been carried out on the performance of such systems [23]. The Herd Navigation® system (Tumba, Sweden), which integrates five sensing systems, including progesterone in milk, was developed for commercial usage in 2008. It detects progesterone levels in milk and recommends insemination times, animals for final pregnancy confirmation, early termination, and cows at risk for cysts and extended anestrus. In Denmark, estrus detection rates of 95–97% have been recorded, with much higher pregnancy rates (up to 42–50%) than traditional approaches [22]. Endocrine regulation is crucial for optimal fertilization throughout the follicular period. Because progesterone production is strongly related to embryonic development from the early stages of pregnancy, adequate monitoring of both pre-ovulatory decline and post-insemination elevation in milk progesterone can be used to detect animals with reduced fertility. Reduced milk progesterone concentrations, for example, around days 4–7 following insemination are related to low fertility and an increased chance of embryonic loss [21]. According to various studies, pregnant cows had higher quantities of milk progesterone in their milk samples for the first week following insemination [11].
Infrared spectroscopy is a fast, inexpensive, and user-friendly technology that can be used for research as well as online and offline milk analyses [1]. Near-infrared spectroscopy (NIRS) has long been used to measure the amount of milk components such as fat, protein, and lactose [24]. Numerous studies demonstrate that a near-infrared spectroscopy approach is a viable method for assessing individual milk progesterone levels. In fact, monitoring progesterone levels in milk is an efficient and cost-effective method for determining a cow’s reproductive status, detecting heat, and diagnosing pregnancy. Cows are milked twice or three times per day under standard dairy practices, implying that milk samples provide information on the current health of the herd/individual and may be collected and tested on a frequent basis without severely compromising the animal’s daily living [1]. In addition, it has been demonstrated that the physiological status of the animal affects the molecular structure of the water in milk; therefore, milk spectra can provide useful information regarding animal health and sickness [24].
3. Breath, Sweat and Saliva Analysis
Researchers have long been interested in disease detection by the identification of volatile organic compounds (VOCs), representing a non-invasive technique. Animals and humans both exhale and excrete VOCs in their secretions with breath, blood, feces, skin, urine, and vaginal discharges [25][26]. Gases such as hydrogen (H2) and methane (CH4), as well as volatile organic molecules such as fatty acids, which can serve as biomarkers for metabolic and pathologic processes, are examples of metabolites found in the breath. VOCs such as ketone bodies, ethanol, methanol, and exogenous compounds are commonly associated with blood glucose levels [27]. VOC analysis has been used to study bovine respiratory sickness, brucellosis, bovine tuberculosis, Johne’s disease, ketoacidosis, and normal rumen physiology in cattle [22][25].
The majority of sweat metabolite analysis biosensors were created with the intention of monitoring human health. These have been put to use to measure lactate levels and salt concentrations, and they have also been made portable (in belt form) to measure sweat [22]. Additionally, this sensor may be modified for use in measuring animal perspiration, particularly as an indicator of physical stress in animals [22][28].
Saliva collection for disease and other biochemical markers of physiological health is an appealing non-invasive alternative to blood sampling [29]. Because saliva contains both local and systemic components, it is a useful source of information regarding systemic processes occurring in the body, allowing for the evaluation of the organism’s physiological or pathological status. However, the use of saliva in the diagnosis of animal illnesses necessitates a detailed examination of its protein composition under various situations [30][31]. The technique is especially helpful for monitoring animals and diagnosing diseases because drawing blood from animals is thought to be a stressor and can affect the biochemical parameters being measured. Salivary biomarkers can be useful for several purposes, including disease early detection and diagnosis, decision support for managing animals, and disease progression monitoring [32]. There are other diagnostic applications for saliva, such as biomarkers from saliva being investigated for the detection of oral cancer [33]. According Mojsym et al., saliva can be a valuable diagnostic sample comprising possible indications of physiological and pathological conditions such as pregnancy status of cattle. Moreover, it can be useful for developing rapid tests from saliva [30]. In buffalo, there are attempts to determine estrous time using saliva biomarkers for more precise insemination planning [34]. Saliva analytes were explored for their relationships with lameness in another investigation. It was also anticipated that cows can change certain particles in their saliva, and some of these may reflect improvements in lameness after therapy [35]. Table 1 shows a summary of milk and other body fluid analysis and its benefits.
Table 1. Body fluid analysis and its benefits.
| Technology | Benefits of Use | Reference |
|---|---|---|
| Milk progesterone | Milk progesterone is a potential non-invasive indicator of reproductive status in dairy cows | [21] |
| Somatic cell count | SCC has proven to be a useful, non-invasive indicator of subclinical mastitis | [16] |
| Breath, Sweat and Saliva analysis | Biomarkers for metabolic and pathologic processes are examples of metabolites found in the breath. VOCs such as ketone bodies, ethanol, methanol, and exogenous compounds are commonly associated with blood glucose levels. Saliva collection is a non-invasive alternative to blood sampling | [3][25] |
4. Wearable Devices for Animals
Portable electronic monitoring devices have the potential to transform intensive, large-scale dairy production by monitoring and managing cows on an individual basis. There has been a notable increase in the number of published research looking into the application of wearable electronic monitoring devices for use in commercial farming environments since the early 2000s [36].
4.1. Head/Muzzle and Noseband Sensors
Three distinct kinds of biosensors can be employed to recognize the jaw movements that characterize cattle grazing behavior. These are electromyography sensors, mechanical/pressure sensors, and acoustic sensors [22]. Cattle grazing behavior necessitates close observation of each cow based on three crucial factors: the cow’s position, an analysis of its posture, and the cow’s motions, particularly its gait and jaw movement [22][37]. The amount of time an animal spends with its head in a downward posture is added to the sensor’s recorded data to calculate grazing time [38]. For instance, continuous observation of jaw motions can reveal information about diurnal grazing habits, animal health disorders, and forage deficiencies [39][40].
Animals adjust their behavior in response to stresses, social changes, and environmental changes, and these can cause illnesses. Because of the labor necessary for the continuous monitoring of big groups of animals, tracking this behavior on a large scale becomes impossible. When combined with proper output interpretations, wearable sensor technologies enable the simultaneous measurement of real-time physiological parameters in a herd on a large scale. As a result, wearable sensor technologies offer an advantage over traditional herd-based systems since data from wearable sensors can be evaluated instantly, allowing for a short reaction time [41]. For example, RumiWatch (RWS; ITIN + HOCH GmbH, Liestal, Switzerland), a noseband sensor that monitors feeding and rumination activity in dairy cows, was designed and tested as an effective scientific monitoring device for automated measurements of rumination behavior and activities. The correlations between direct observations and sensor readings reveal that the RumiWatch noseband sensor was effectively designed and validated as a scientific monitoring device for the automated detection of rumination and eating behaviors in stable-fed dairy cows [40][42]. Other examples of commercial sensors and their detected analytes are presented in Table 2. According to the findings of the study, the rumination and feeding activity monitoring system is an effective tool for predicting calving time under farm conditions. It was found that in primiparous and multiparous cows, lying bouts increased but rumination chews declined while predicting calving. Logistic regression and ROC analysis were used to assess the sensitivity (Se) and specificity (Sp) for predicting the commencement of calving within 3 h (Se = 88.9%, 85% and Sp = 93.3%, 74% for multiparous and primiparous cows, respectively) [43].
Table 2. Information about some of the commercial sensors.
| Sensor | Method | Detected Analytes | Reference |
|---|---|---|---|
| RumiWatch (Itin + Hoch GmbH, Liestal, Switzerland) | The RW system comes with software for controlling the sensor (RW Manager) and studying unprocessed data (RW Converter). The RW sensors, which include a noseband pressure sensor, a three-axis accelerometer to track three-dimensional head motions, and a data logger, are built into a halter that fits the head of each particular animal. The noseband pressure sensor, which is mounted in a belt on the animal’s nose bridge, is connected to a tube filled with propylene glycol to detect jaw movements. As the animal moves its jaw, pressure within the tube varies, and this information is recorded with a 10 Hz resolution. Approximately 100 days of raw data logging were covered by the battery life. | Different pressure signatures of jaw motions, which are then detected and categorized into prehension bites, mastication chews, and rumination chews | [38] |
| Ear tag–based accelerometer system (Smartbow GmbH, Weibern, Austria) | The ear tag has an acceleration sensor, a radio chip, and a temperature sensor for calibration and it can monitor rumination and detect estrus and localization. | Rumination, estrus, and current localization | [44] |
| MoonSyst (Moonsyst International Ltd.: P.O. Box 1329, Kinsale, Co., Cork, Republic of Ireland) | System captures rumen data in real time. The bolus is meant to be readily ingested and will remain in the rumen (particularly the reticulum) throughout the animal’s life. System sends data from the animal to specialized cloud-based servers via a communication gateway. Farmers may use the Mooncloud software application to view information from anywhere, anytime. The bolus can be used on animals weighing more than 350 kg. Once implanted, the bolus interacts with a gateway over a large geographical region. | Heats, monitor health conditions, activity, rumen temperature and movement | [45] |
| SmaXtec (SmaXtec animal care GmbH, Graz, Austria) | The rumen bolus accurately monitors direct, informative values inside cows’ reticulum. The boluses are given once and require no further maintenance. The data from the boluses are read out by the readout devices with an integrated Internet connection and promptly transferred to the cloud. The pH and temperature variation data are gathered with an analogue-to-digital converter (A/D converter) and stored in an external memory chip. This indwelling system may be simply orally supplied to an adult cow due to its dimensions (length: 12 cm, width: 3.5 cm, weight: 210 g), and its particular construction makes it shock-proof and resistant to rumen fluid. | pH, ruminal temperature, cow activity, drinking, eating, rumen behavior | [46] |
| Body Condition Score Camera (DeLaval, International AB, Tumba, Sweden) | Body condition score system is based on a 3D camera that records certain areas of the animal: from above, the rear part of the back from the short ribs to the tail end. When a cow moves in front of the camera, the system recognizes the movement and records photographs of the cow; it then selects the best image of the cow from the video clip. The 3D camera employs light coding technology to project a pattern of infrared ray dots on the cow’s back. Following that, the distances between these specific dots are measured; the company claims that a 3D picture of the back is created, and an algorithm translates the image information into a body condition score. | BCS | [11] |
| CattleEye (Cattle Eye Ltd., Belfast, UK) | Camera is above the exit gate of a milking parlor. It records video of each cow as it exits the milking parlor. If a sort of gate or RFID system provides ID information, use it. Artificial intelligence systems in the cloud analyze video to uniquely identify the cows and track their wellness, among other things. System allows tracking the health and performance of cows in real time. It includes a dashboard that monitors and visualizes a variety of vital indicators at the herd and cow levels. | Cow identification | [47] |
| Cainthus (© 2022 Ever.Ag, Frisco, TX, USA) | Smart camera system that monitors animal behavior and farm activities 24 h a day, seven days a week, 365 days a year. It is artificial intelligence that converts visual input from cameras into real-time insights. These insights are provided daily on any farm device, phone, tablet, or computer. The information provided is accurate and unbiased. This technique is easily scalable, does not require any hardware on the cows, and requires extremely minimal maintenance in comparison to other solutions. |
Animal behavior | [48] |
| BROLIS Herdline (Vilnius, Lithuania) | The analyzer examines the composition of each cow’s milk during each milking. This “mini-spectroscope” is installed in the milking stalls or milking robot in the milk line and does not use additional reagents and does not require special maintenance. The analysis of protein, fat, lactose, and electrical conductivity provides a proper evaluation of the health, productivity, and economic efficiency of dairy cattle. The data collected during milking are processed in real time and can be viewed using the BROLIS HerdLine application. |
Milk fat, protein, lactose, milk electrical conductivity | [12] |
| HeatWatch (HeatWatch® DDx, Inc., Denver, CO, USA) | A tiny radionic transmitter is linked to a pressure sensor in a stiff plastic box implanted in a nylon packaging that is glued to the cow’s tail hair in the sacral region. The device is activated by the weight of the mounting animal for a minimum of 2 seconds, after which the transmitter sends the breeding approval signal to the system along with the animal’s identification. In general, this device’s assessed performance ranges from 37% to 94%. | Heat detection | [49] |
4.2. Motion, Movement, and Behavior Sensors
Activity can be reduced in cattle afflicted with lameness or diseases such as bovine respiratory diseases (BRD). Energy conservation for immune system metabolic costs and indirect effects of fever and inflammatory responses to infection were proposed as biological underpinnings for reduced activity in ill animals [50]. There are plenty of technologies for motion, movement, and behavior analysis, such as accelerometer, pedometers, and global positioning system (GPS).
Accelerometers have been used in dairy farming systems for the detection of diseases such as mastitis and to detect estrus and locomotion problems [50]. Accelerometer reading changes can also be used to generate a benchmark level of activity, which can subsequently be recorded as calculated step counts or other movement indices such as activity ratios. Accelerometers have acquired popularity in beef cattle research because they allow for the continuous and long-term study of an animal’s mobility and behavior [50]. Moreover, ear-mounted sensors accurately identified grazing, standing, and walking in sheep with 94%, 95%, and 99% accuracy, respectively [51]. Commercially available accelerometer devices include the IceTag and IceQube products manufactured by IceRobotics, Ltd. (Edinburgh, Scotland, UK), designed and validated for use in cattle. Other commercial accelerometer products designed for use in cattle include CowScout (GEA Group, Dusseldorf, Germany), SCR (Allflex, Madison, WI, USA), Pedometer Plus (Madero Dairy Systems, Houston, TX, USA), GYUHO SaaS (Fujitsu, Fukuoka, Japan), and GP1 SENSR (Reference LLC, Elkader, IA, USA). Another accelerometer device that has been successfully used to quantify cattle behavior, the HOBO Pendant G (Onset Computer Corp., Bourne, MA, USA), requires the user to build a method of leg attachment, and data management is more complicated [50]. When compared to the accuracy reached with these devices (more than 90%), the accuracy of visual human observation is significantly lower [52].
Understanding how grazing animals migrate throughout pastures and what they do in each region is essential for developing management plans that will maximize the potential productivity of grazing systems and limit their negative impacts on the environment (nutrient losses to water and gaseous emissions). Using real-time global positioning system (GPS) tracking and biologging technologies, it is possible to perform remote monitoring of animals to look for any indications of illness or concerns regarding their well-being [53]. Animals on livestock farms can be monitored for their activity levels, which can provide useful information about the animals’ overall health and degree of care [54]. The global positioning system, radio tracking, and wireless local area network are now the most important technologies for monitoring livestock in the field; however, there are a few more tools (such as Bluetooth and ultrasound) that can be employed for indoor monitoring [53]. GPS collars equipped with activity sensors enable the distinction between foraging sites and those used for other activities such as sleeping or travelling [38][55][56]. Diverse studies conducted over the past decade have proved the utility of GPS telemetry devices for analyzing the behavior of cattle when combined with other devices/sensors. Combining GPS collars with activity sensors results in an effective method for tracking the whereabouts of grazing animals and determining animal behavior simultaneously. Real-time location systems have been created to pinpoint the position of an object within a particular area [57]. Although little research has been conducted on the behavior and movement of lame dairy cows on pasture, GPS technology may be useful in enhancing pasture-based systems’ automatic lameness diagnosis. According to Riaboff et al., severely lame cows spend 4.5 times less time grazing and nearly twice as much time resting in the laying position than their sound counterparts [58]. Additionally, GPS is used to forecast the behavior of ruminants and locate their location in pastures. This geolocation technique appears promising for identifying animals with a high frequency and a low mistake rate, despite the GPS’s inadequacy for forecasting behavior in a robust manner. Geolocated behaviors are especially intriguing for investigating changes in behavior connected to demanding events. The usage of data from accelerometers and GPS devices together is an approach that could prove to be both intriguing and beneficial in the process of researching how cows interact with their surroundings. These challenging scenarios can include heat stress, physical stress, resource depletion, restricted access to pasture, and other similar situations [59]. These characteristics could be used to improve the efficacy of existing lameness detection sensors in pasture-based systems [58]. Table 3 gives a brief summary of wearable sensors.
Table 3. Wearable sensors and their benefits.
| Technology | Benefits of Use | Reference |
|---|---|---|
| Head/muzzle and noseband sensors | Noseband sensor was designed and validated as a scientific monitoring device for the automated detection of rumination and eating behaviors. It can be executed without contact with the animal. | [40] |
| Motion, movement, and behavior sensors | Accelerometers, pedometers, and GPS tracking all can be used to monitor animal behavior. Active time can predict heat; prolonged laying time can signal diseases such as mastitis, ketosis, and lameness. GPS helps to locate animals on the farm. | [50][54][59] |
There are GPS systems that allow users to track and confine animals. Special attention has been paid to a solar-powered GPS collar-based virtual fence system (NoFence, Beatnfjordsra, Norway). The GPS position data collected by the collar are shown on an app that the farmer uses to create a grazing border map for cattle, sheep, or goats. When the animal gets close to the virtual boundary, the collar will begin to emit warning audio stimuli at a volume of forty decibels and a rising frequency of two to four thousand hertz. This will give the animal the time to change course to avoid such stimuli. When an animal passes the virtual barrier, it is considered to have “escaped”, and the auditory and the electric shock are turned off. Additionally, a push notification is delivered to the farmer’s mobile app when the animal has crossed the boundary [60]. GPS also can be promising in heat detection. Sheep estrus can be detected with global navigation satellite systems (GNSS) by monitoring a surge in activity levels followed by a return to “normal” patterns of behavior [51].
Pedometer
In animals, a pedometer (step counter) is a proven recording method for determining movement activity. Previously, it was mostly utilized for estrus detection [3]. Pedometers objectively measure an animal’s total number of steps and total distance traveled using an algorithm that calculates the steps. While pedometers are relatively simple to deploy and operate, the number of steps taken by each ruminant varies significantly depending on the day and ambient conditions. There could be a link between cattle distance traveled and stressful and unpleasant procedures; one study found that calves took fewer steps for four days after castration, while another found a link between calves’ stress and the number of steps they took after castration [57]. Other observations are made while recording active or lying behavior. The method is useful for the early identification of lameness in dairy herds. In one investigation, pedometers were used to detect lame calves before clinical indications appeared. Individual cows were examined and monitored, and it was shown that 92% of the cows acquired obvious lameness. When cattle were monitored using a pedometer, their hoof activity was reduced by at least 15% several days before the start of clinical lameness. The researchers concluded that pedometers are a beneficial tool for the early detection and treatment of the vast majority of cases of developing lameness [61].
5. Other Analyzers: BCS Camera, Infrared Thermography, Sensors of Bolus
Thermography and its measured analytes and other electronic devices are reviewed in Table 4.
Table 4. Other sensors and their benefits.
| Technology | Benefits of Use | Reference |
|---|---|---|
| Infrared Thermography | Determine thermal abnormalities in animals by identifying a rise or fall in the surface temperature of skin. Infrared thermography is a noninvasive method that monitors infrared radiation emitted from the body. Inflammation, stress, calving, and heat can be evaluated. Thermography can detect physiological changes before they emerge as clinical symptoms. | [62][63][64] |
| Bolus Sensors | Wireless intraruminal boluses without constant contact, can measure and analyze ruminal and eating behavior, examine ruminal pH. | [21][57][65] |
| Body Condition Score Cameras | Tracking BCS can help reduce postpartum disease percent; it helps to notice obese or poor health animals. When it comes to production and reproduction, lower calving BCS is connected with lower rates, while greater calving BCS is associated with an increased risk of metabolic diseases | [66][67][68][69] |
| Cattle Face Recognition | Face analysis can help to identify pain, unwell animals, locate, identify, and select animals on the farm. | [70][71] |
| Electronic Nose for Estrus Detection | Can detect estrus by direct sampling of odor from the perineal headspace. | [72] |
This entry is adapted from the peer-reviewed paper 10.3390/ani13050780
References
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An Overview on the Use of Near Infrared Spectroscopy (NIRS) on Farms for the Management of Dairy Cows. Agriculture 2021, 11, 296.
- Eckelkamp, E.A. Invited Review: Current state of wearable precision dairy technologies in disease detection. Appl. Anim. Sci. 2019, 35, 209–220.
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. BioSens. Res. 2017, 12, 15–29.
- Nogami, H.; Okada, H.; Miyamoto, T.; Maeda, R.; Itoh, T. Wearable Wireless Temperature Sensor Nodes Appressed to Base of a Calf’s Tail. Sens. Mater. 2014, 26, 539–545.
- Van Nuffel, A.; Zwertvaegher, I.; Van Weyenberg, S.; Pastell, M.; Thorup, V.; Bahr, C.; Sonck, B.; Saeys, W. Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior. Animals 2015, 5, 861–885.
- Badwolf. CowAlert|Use Sensors to Manage Your Herd. IceRobotics. Available online: https://www.icerobotics.com/cowalert/ (accessed on 14 November 2022).
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A Wearable Platform for Harvesting and Analysing Sweat Sodium Content. Electroanalysis 2016, 28, 1283–1289.
- Heikenfeld, J. Technological leap for sweat sensing. Nature 2016, 529, 475–476.
- U-Motion®—Monitor Your Herd’s Behavior. Available online: http://desamis.co.jp/en/ (accessed on 14 November 2022).
- Stone, A.E. Symposium review: The most important factors affecting adoption of precision dairy monitoring technologies. J. Dairy Sci. 2020, 103, 5740–5745.
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Baumgartner, W. Relation of Automated Body Condition Scoring System and Inline Biomarkers (Milk Yield, β-Hydroxybutyrate, Lactate Dehydrogenase and Progesterone in Milk) with Cow’s Pregnancy Success. Sensors 2021, 21, 1414.
- Diagnostic Value of Milk Fat. BROLIS HerdLine. Available online: https://brolisherdline.com/milk-fat/ (accessed on 3 December 2022).
- Luo, T.; Steeneveld, W.; Nielen, M.; Zanini, L.; Zecconi, A. Linear Mixed-Effects Model to Quantify the Association between Somatic Cell Count and Milk Production in Italian Dairy Herds. Animals 2023, 13, 80.
- Neculai-Valeanu, A.-S.; Ariton, A.-M. Udder Health Monitoring for Prevention of Bovine Mastitis and Improvement of Milk Quality. Bioengineering 2022, 9, 608.
- Wang, Y.; Kang, X.; He, Z.; Feng, Y.; Liu, G. Accurate detection of dairy cow mastitis with deep learning technology: A new and comprehensive detection method based on infrared thermal images. Animal 2022, 16, 100646.
- Carvalho-Sombra, T.C.; Fernandes, D.D.; Bezerra, B.M.; Nunes-Pinheiro, D.C. Systemic inflammatory biomarkers and somatic cell count in dairy cows with subclinical mastitis. Vet. Anim. Sci. 2021, 11, 100165.
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562–577.
- Balaine, L.; Dillon, E.J.; Läpple, D.; Lynch, J. Can technology help achieve sustainable intensification? Evidence from milk recording on Irish dairy farms. Land Use Policy 2020, 92, 104437.
- Martins, S.A.M.; Martins, V.C.; Cardoso, F.A.; Germano, J.; Rodrigues, M.; Duarte, C.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Biosensors for On-Farm Diagnosis of Mastitis. Front. Bioeng. Biotechnol. 2019, 7, 186.
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population—A review. Vet. Q 2019, 39, 76–94.
- Zachut, M.; Šperanda, M.; de Almeida, A.M.; Gabai, G.; Mobasheri, A.; Hernández-Castellano, L.E. Biomarkers of fitness and welfare in dairy cattle: Healthy productivity. J. Dairy Res. 2020, 87, 4–13.
- Neethirajan, S.; Tuteja, S.K.; Huang, S.-T.; Kelton, D. Recent advancement in biosensors technology for animal and livestock health management. Biosens. Bioelectron. 2017, 98, 398–407.
- Rutten, C.J.; Velthuis, A.G.J.; Steeneveld, W.; Hogeveen, H. Invited review: Sensors to support health management on dairy farms. J. Dairy Sci. 2013, 96, 1928–1952.
- Muncan, J.; Miyazaki, M.; Kuroki, S.; Ikuta, K.; Tsenkova, R. Adaptive Spectral Model for abnormality detection based on physiological status monitoring of dairy cows. Talanta 2023, 253, 123893.
- Burciaga-Robles, L.O.; Holland, B.P.; Step, D.L.; Krehbiel, C.R.; McMillen, G.L.; Richards, C.J.; Sims, L.E.; Jeffers, J.D.; Namjou, K.; McCann, P.J. Evaluation of breath biomarkers and serum haptoglobin concentration for diagnosis of bovine respiratory disease in heifers newly arrived at a feedlot. Am. J. Vet. Res. 2009, 70, 1291–1298.
- Garner, C.E.; Smith, S.; Bardhan, P.K.; Ratcliffe, N.M.; Probert, C.S.J. A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1171–1173.
- Leopold, J.H.; van Hooijdonk, R.T.; Sterk, P.J.; Abu-Hanna, A.; Schultz, M.J.; Bos, L.D. Glucose prediction by analysis of exhaled metabolites—A systematic review. BMC Anesthesiol. 2014, 14, 46.
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560.
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371.
- Mojsym, W.; Wawrzykowski, J.; Jamioł, M.; Chrobak, Ł.; Kankofer, M. Comparative Analysis of Saliva and Plasma Proteins Patterns in Pregnant Cows—Preliminary Studies. Animals 2022, 12, 2850.
- Global Survey of the Bovine Salivary Proteome: Integrating Multidimensional Prefractionation, Targeted, and Glycocapture Strategies. Journal of Proteome Research. Available online: https://pubs.acs.org/doi/10.1021/pr200516d (accessed on 4 January 2023).
- Malon, R.S.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. BioMed Res. Int. 2014, 2014, 962903.
- Gautam, S.S.; Singh, R.P.; Karsauliya, K.; Sonker, A.K.; Reddy, P.J.; Mehrotra, D.; Gupta, S.; Singh, S.; Kumar, R.; Singh, S.P. Label-free plasma proteomics for the identification of the putative biomarkers of oral squamous cell carcinoma. J. Proteom. 2022, 259, 104541.
- Singh, L.K.; Pandey, M.; Baithalu, R.K.; Fernandes, A.; Ali, S.A.; Jaiswal, L.; Pannu, S.; Bhatia, N.; Mohanty, T.K.; Kumaresan, A.; et al. Comparative Proteome Profiling of Saliva Between Estrus and Non-Estrus Stages by Employing Label-Free Quantitation (LFQ) and Tandem Mass Tag (TMT)-LC-MS/MS Analysis: An Approach for Estrus Biomarker Identification in Bubalus bubalis. Front. Genet. 2022, 13, 969.
- Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Želvytė, R.; Tecles, F.; Rubio, C.P. Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals 2020, 10, 2078.
- Hendriks, S.J.; Phyn, C.V.; Huzzey, J.M.; Mueller, K.R.; Turner, S.A.; Donaghy, D.J.; Roche, J.R. Graduate Student Literature Review: Evaluating the appropriate use of wearable accelerometers in research to monitor lying behaviors of dairy cows. J. Dairy Sci. 2020, 103, 12140–12157.
- Andriamandroso, A.; Bindelle, J.; Mercatoris, B.; Lebeau, F. A review on the use of sensors to monitor cattle jaw movements and behavior when grazing. Biotechnol. Agron. Société Environ. 2016, 20.
- Raynor, E.J.; Derner, J.D.; Soder, K.J.; Augustine, D.J. Noseband sensor validation and behavioural indicators for assessing beef cattle grazing on extensive pastures. Appl. Anim. Behav. Sci. 2021, 242, 105402.
- Linnane, M.I.; Brereton, A.J.; Giller, P.S. Seasonal changes in circadian grazing patterns of Kerry cows (Bos taurus) in semi-feral conditions in Killarney National Park, Co. Kerry, Ireland. Appl. Anim. Behav. Sci. 2001, 71, 277–292.
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Rutkaukas, A.; Šertvytytė, G.; Baumgartner, W. Identification of Changes in Rumination Behavior Registered with an Online Sensor System in Cows with Subclinical Mastitis. Vet. Sci. 2022, 9, 454.
- Pezeshki, A.; Stordeur, P.; Wallemacq, H.; Schynts, F.; Stevens, M.; Boutet, P.; Peelman, L.J.; De Spiegeleer, B.; Duchateau, L.; Bureau, F.; et al. Variation of inflammatory dynamics and mediators in primiparous cows after intramammary challenge with Escherichia coli. Vet. Res. 2011, 42, 15.
- Zehner, N.; Umstätter, C.; Niederhauser, J.J.; Schick, M. System specification and validation of a noseband pressure sensor for measurement of ruminating and eating behavior in stable-fed cows. Comput. Electron. Agric. 2017, 136, 31–41.
- Fadul, M.; Bogdahn, C.; Alsaaod, M.; Hüsler, J.; Starke, A.; Steiner, A.; Hirsbrunner, G. Prediction of calving time in dairy cattle. Anim. Reprod. Sci. 2017, 187, 37–46.
- Herd Monitoring Software|SMARTBOW. Available online: https://www.smartbow.com/en/Home.aspx (accessed on 23 November 2022).
- Moonsyst. Available online: https://moonsyst.com/home (accessed on 3 December 2022).
- How It Works. Available online: https://smaxtec.com/en/function/ (accessed on 4 January 2023).
- CattleEye|Autonomous Livestock Monitoring. Available online: https://cattleeye.com/ (accessed on 3 December 2022).
- Technology. Cainthus. Available online: https://www.cainthus.com/technology (accessed on 3 December 2022).
- Saint-Dizier, M.; Chastant-Maillard, S. Potential of connected devices to optimize cattle reproduction. Theriogenology 2018, 112, 53–62.
- Richeson, J.T.; Lawrence, T.E.; White, B.J. Using advanced technologies to quantify beef cattle behavior1. Transl. Anim. Sci. 2018, 2, 223–229.
- Lewis Baida, B.E.; Swinbourne, A.M.; Barwick, J.; Leu, S.T.; van Wettere, W.H.E.J. Technologies for the automated collection of heat stress data in sheep. Anim. Biotelemetry 2021, 9, 4.
- Lovarelli, D.; Bacenetti, J.; Guarino, M. A review on dairy cattle farming: Is precision livestock farming the compromise for an environmental, economic and social sustainable production? J. Clean. Prod. 2020, 262, 121409.
- Rivero, M.J.; Grau-Campanario, P.; Mullan, S.; Held, S.D.E.; Stokes, J.E.; Lee, M.R.F.; Cardenas, L.M. Factors Affecting Site Use Preference of Grazing Cattle Studied from 2000 to 2020 through GPS Tracking: A Review. Sensors 2021, 21, 2696.
- Cabezas, J.; Yubero, R.; Visitación, B.; Navarro-García, J.; Algar, M.J.; Cano, E.L.; Ortega, F. Analysis of Accelerometer and GPS Data for Cattle Behaviour Identification and Anomalous Events Detection. Entropy 2022, 24, 336.
- Augustine, D.J.; Derner, J.D. Assessing Herbivore Foraging Behavior with GPS Collars in a Semiarid Grassland. Sensors 2013, 13, 3711–3723.
- Ganskopp, D.C.; Bohnert, D.W. Landscape nutritional patterns and cattle distribution in rangeland pastures. Appl. Anim. Behav. Sci. 2009, 116, 110–119.
- Neethirajan, S. Transforming the Adaptation Physiology of Farm Animals through Sensors. Animals 2020, 10, 1512.
- Riaboff, L.; Relun, A.; Petiot, C.-E.; Feuilloy, M.; Couvreur, S.; Madouasse, A. Identification of discriminating behavioural and movement variables in lameness scores of dairy cows at pasture from accelerometer and GPS sensors using a Partial Least Squares Discriminant Analysis. Prev. Vet. Med. 2021, 193, 105383.
- Riaboff, L.; Couvreur, S.; Madouasse, A.; Roig-Pons, M.; Aubin, S.; Massabie, P.; Chauvin, A.; Bédère, N.; Plantier, G. Use of Predicted Behavior from Accelerometer Data Combined with GPS Data to Explore the Relationship between Dairy Cow Behavior and Pasture Characteristics. Sensors 2020, 20, 4741.
- Caja, G.; Castro-Costa, A.; Salama, A.A.K.; Oliver, J.; Baratta, M.; Ferrer, C.; Knight, C.H. Sensing solutions for improving the performance, health and wellbeing of small ruminants. J. Dairy Res. 2020, 87, 34–46.
- LokeshBabu, D.S.; Jeyakumar, S.; Vasant, P.J.; Sathiyabarathi, M.; Manimaran, A.; Kumaresan, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Kataktalware, M.A.; et al. Monitoring foot surface temperature using infrared thermal imaging for assessment of hoof health status in cattle: A review. J. Therm. Biol. 2018, 78, 10–21.
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16.
- Alsaaod, M.; Schaefer, A.L.; Büscher, W.; Steiner, A. The Role of Infrared Thermography as a Non-Invasive Tool for the Detection of Lameness in Cattle. Sensors 2015, 15, 14513–14525.
- Kang, X.; Zhang, X.D.; Liu, G. A Review: Development of Computer Vision-Based Lameness Detection for Dairy Cows and Discussion of the Practical Applications. Sensors 2021, 21, 753.
- Nogami, H.; Arai, S.; Okada, H.; Zhan, L.; Itoh, T. Minimized Bolus-Type Wireless Sensor Node with a Built-In Three-Axis Acceleration Meter for Monitoring a Cow’s Rumen Conditions. Sensors 2017, 17, 687.
- Mullins, I.L.; Truman, C.M.; Campler, M.R.; Bewley, J.M.; Costa, J.H.C. Validation of a Commercial Automated Body Condition Scoring System on a Commercial Dairy Farm. Animals 2019, 9, 287.
- Qiao, Y.; Kong, H.; Clark, C.; Lomax, S.; Su, D.; Eiffert, S.; Sukkarieh, S. Intelligent perception for cattle monitoring: A review for cattle identification, body condition score evaluation, and weight estimation. Comput. Electron. Agric. 2021, 185, 106143.
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801.
- Ruchay, A.; Kober, V.; Dorofeev, K.; Kolpakov, V.; Miroshnikov, S. Accurate body measurement of live cattle using three depth cameras and non-rigid 3-D shape recovery. Comput. Electron. Agric. 2020, 179, 105821.
- Gaber, T.; Tharwat, A.; Hassanien, A.E.; Snasel, V. Biometric cattle identification approach based on Weber’s Local Descriptor and AdaBoost classifier. Comput. Electron. Agric. 2016, 122, 55–66.
- Yao, L.; Hu, Z.; Liu, C.; Liu, H.; Kuang, Y.; Gao, Y. Cow face detection and recognition based on automatic feature extraction algorithm. In Proceedings the ACM Turing Celebration Conference—China; ACM: Chengdu, China, 2019; pp. 1–5.
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Graboski, A.M.; De Mello Brandão, H.; de Carvalho, B.C.; Bellini, J.L.; de Paula Herrmann, P.S. Volatile compounds monitoring as indicative of female cattle fertile period using electronic nose. Sens. Actuators B Chem. 2019, 282, 609–616.
This entry is offline, you can click here to edit this entry!
