Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
Primary cutaneous lymphomas constitute a heterogeneous group of disorders characterized by monoclonal proliferations of lymphocytes with infiltration primarily involving the skin, modified skin appendages and certain mucosal sites. Primary cutaneous lymphomas are a heterogeneous group of disorders and a current area of unmet need in dermato-oncology due to the limited options available for advanced cases.
- cutaneous lymphoma
- monoclonal antibodies
- rituximab
- brentuximab vedotin
1. Classification
In the Western world, cutaneous T cell lymphoma (CTCLs) represent approximately 75 to 80% of all primary cutaneous lymphomas [28,29]. Specifically, mycosis fungoides (MF), Sezary syndrome (SS), and primary cutaneous CD30+ lymphoproliferative disorders (LPD) account for 55, 5 and 30% of CTCL, respectively.
MF represents almost 55% of cases of CTCL; three peculiar variants with distinctive clinicopathologic features are included within this diagnosis: folliculotropic MF, pagetoid reticulosis, and granulomatous slack skin. SS accounts for only 5% of cases and is a leukemic type of CTCL presenting with erythroderma and peripheral lymphadenopathy. The detection of neoplastic T cells (Sezary cells) in skin, lymph nodes, and peripheral blood is characteristic of SS [30,31].
Primary cutaneous CD30+ LPD is the term for a group of disorders that represent approximately 30% of CTCL overall. This group includes primary cutaneous anaplastic large cell lymphoma (PCALCL), lymphomatoid papulosis (LyP), and borderline cases.
Additional entities are: adult T cell leukemia-lymphoma, a peripheral T cell neoplasm associated with the human T cell leukemia virus 1 (HTLV-1); subcutaneous panniculitis-like T cell lymphoma, which has an alpha-beta T cell phenotype associated with an indolent biologic behavior; and extranodal NK/T cell lymphoma, nasal type, which is in almost all cases an Epstein–Barr virus (EBV)-positive lymphoma composed of small, medium, or large cells (with NK or, rarely, cytotoxic T cell phenotype).
Finally, rare subtypes of primary cutaneous peripheral T cell lymphoma have been recognized, including provisionally classified entities: primary cutaneous gamma-delta T cell lymphoma, primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma, primary cutaneous acral CD8+ T cell lymphoma, primary cutaneous CD4+ small/medium T cell LPD [29].
2. Pathophysiology of T Cells
Local immune and inflammatory responses in the skin are dependent on the migratory abilities of T cells that are tightly regulated by lymphocyte-endothelial interactions [32]. Homing to the cutaneous compartment is a multistep process. Adhesion between lymphocytes and endothelial cells is mediated by molecules expressed on the cell surface and promoted by the secretion of chemokines which occurs in the context of local inflammation. Extravasation begins when the cutaneous lymphocyte antigen (CLA) on the lymphocyte interacts with E-selectin on activated endothelium; this initial tethering brings the cell close to the stimuli of local chemokines such as CCR4, which activate surface integrins. The latter provide firm anchoring to intercellular adhesion molecules on the endothelium [33]. Immune cells expressing the specific homing receptor CLA include approximately 15% of memory T cells as well as NK cells, monocytes, granulocytes, and a small percentage of B cells [34,35,36]. Owing to the key role of CLA for cutaneous homing, this surface molecule is expressed on malignant cells across a variety of primary cutaneous lymphomas [37].
CTCL are generally derived from memory subsets, mainly CD4+, of T cells that are associated with the expression of CLA together with CCR4 chemokine receptor [38]. This is the typical homing signature of MF cells, accounting for the prolonged localization of MF to specific skin sites [16,39]. Conversely, malignant T cells in SS, a CTCL entity closely related to MF, co-express L-selectin and CCR7, that are the lymph node homing signals of central memory T cells, together with CLA and CCR4 [40]. Additionally, when MF spreads to the lymph nodes CCR7 is expressed and skin homing receptors are downregulated [41]. Apart from MF and SS, CLA has been detected in CD30+ LPDs and in other rare subtypes of CTCL, including primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma [37]. Finally, though its expression has been reported in isolated cases of primary cutaneous B cell lymphoma, the involvement of CLA in the genesis of malignant B cells is likely marginal [37].
3. Distinctive Molecular Alterations of CTCL
Alternations of cellular signaling pathways and imbalances of the skin immune microenvironment have been identified to be at the basis of CTCL. Chromosome translocations leading to activation of oncogenes, deletions or mutations inactivating tumor suppressor genes, and point mutations affecting the epigenetic control of transcription or translation have been increasingly recognized.
The pathogenesis of malignancy in general is a complex process in which increasing genetic damage affects proto-oncogenes and tumor suppressor genes; lymphomagenesis is no exception to this rule. In many subtypes of lymphoma, the genome is characterized by few non-random balanced chromosomal translocations and additional unbalanced alterations that usually accumulate during disease progression. These lesions may be responsible for the activation of oncogenes, while specific deletions suggest inactivation of tumor suppressor genes. However, the spectrum of somatic mutations associated with CTCL is only partially understood. Genome sequencing approaches have been employed to investigate disease pathways that undergo somatic mutation in CTCL highlighting the role of MAPK, NF-kB, PI3K, and TCR; genes related to functions of immune surveillance and RNA splicing have been additionally implicated [42,43].
Recent investigations have demonstrated the importance of MAPK, PI3K/Akt, JAK/STAT, NF-kB, TCR and TLR downstream signaling to allow survival in CTCL. These studies show that cell signaling is differentially altered in different stages and cell populations of CTCL. Specifically, JAK/STAT signaling dysregulation has been described in both early and advanced disease. In early CTCL, activation of STAT signaling is largely dependent on IL-2, IL-7, IL-15 [44].
Different steps of the TCR signaling pathway, from complex formation to activation of transcription factors, also showed abnormalities in the immune microenvironment of CTCL [45].
As far as MF is concerned, its pathophysiology is still incompletely understood but specific molecular pathways have been implicated: TCR and JAK-STAT signaling, RNA splicing and epigenetic control. An increased MAPK signaling due to gain of function mutations in B-RAF and MAPK3 has been demonstrated in subjects with MF [46]. The key proteins in the PI3K/Akt pathway—namely, Akt, mTOR, p70S6K—have been associated with advanced MF stage [47].
Finally, a correlation of CTCL with environmental and occupational exposure to solvents and chemicals has been hypothesized but robust evidence to support this relationship is lacking. In the case of MF, it has been demonstrated that UV genetic changes are associated with altered cell signaling and changes in the skin microenvironment, with gain of function in proto-oncogenes, loss of function in tumor suppressors and adhesion proteins [48].
The currently available antibody-directed treatments for cutaneous lymphoma are summarized in Figure 1. Novel investigative strategies for drug discovery in CTCL will be provided by targeting the key proteins of signaling pathways that stimulate malignant T cells and firing up antitumor immune responses in the CTCL microenvironment [49].
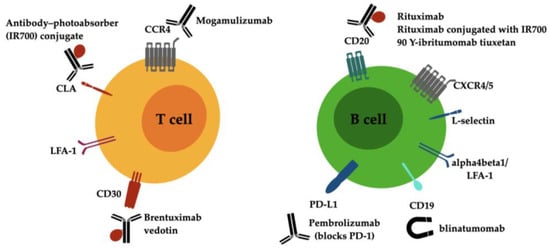
Figure 1. Drug targets on malignant lymphocytes of T and B cell cutaneous lymphoma and current antibody-directed treatment options. Abbreviations: CCR4 C-C chemokine receptor type 4, CD, cluster of differentiation; CLA, cutaneous lymphocyte antigen; CXCR4/5, C-X-C chemokine receptor type 4/5; LFA-1, lymphocyte function-associated antigen 1 (alphaLbeta2 integrin).
This entry is adapted from the peer-reviewed paper 10.3390/antib12010021
This entry is offline, you can click here to edit this entry!
