The capacity of the skin to heal a wound is the result of a highly intricate process that involves several different processes, such as vascular response, blood coagulation, fibrin network creation, re-epithelialisation, collagen maturation, and connective tissue remodelling. Wound healing may be controlled with topical antiseptics, topical antibiotics, herbal remedies, and cellular initiators. In order to effectively eradicate infections and shorten the healing process, contemporary antimicrobial treatments that include antibiotics or antiseptics must be investigated. A variety of delivery systems were described, including innovative delivery systems, hydrogels, microspheres, gold and silver nanoparticles, vesicles, emulsifying systems, nanofibres, artificial dressings, three-dimensional printed skin replacements, dendrimers and carbon nanotubes. It may be inferred that enhanced local delivery methods might be used to provide wound healing agents for faster healing of skin wounds.
- wound
- physiology of wound healing
- strategies towards wound healing
- local delivery systems
1. Introduction
A wound is the result of the “disruption of normal anatomic structure and function,” claims the Wound Healing Society. Depending on how long a wound takes to heal, attention might be given to the acute and chronic kinds. Acute wounds are typically treated effectively with a good possibility of success within a few weeks and are primarily caused by mechanical trauma or surgical procedures [1]. The location, size, depth, and type of an acute wound all affect its nature. The chain of events necessary for wound healing can be disrupted by a variety of disease processes, leading to chronic, non-healing wounds that cause the patient great suffering and need a tremendous number of resources from the medical system. The coagulation cascade, inflammatory pathways, and the cellular components of the immune system are all activated during wound healing, which causes a significant modification of all skin compartments [2]. For expediting in vivo wound healing and minimising scar formation, cellular scaffolds containing fibroblasts, keratinocytes, stem/progenitor cells, or reprogrammed cells have shown promising outcomes.
2. Physiology of Wound Healing Process
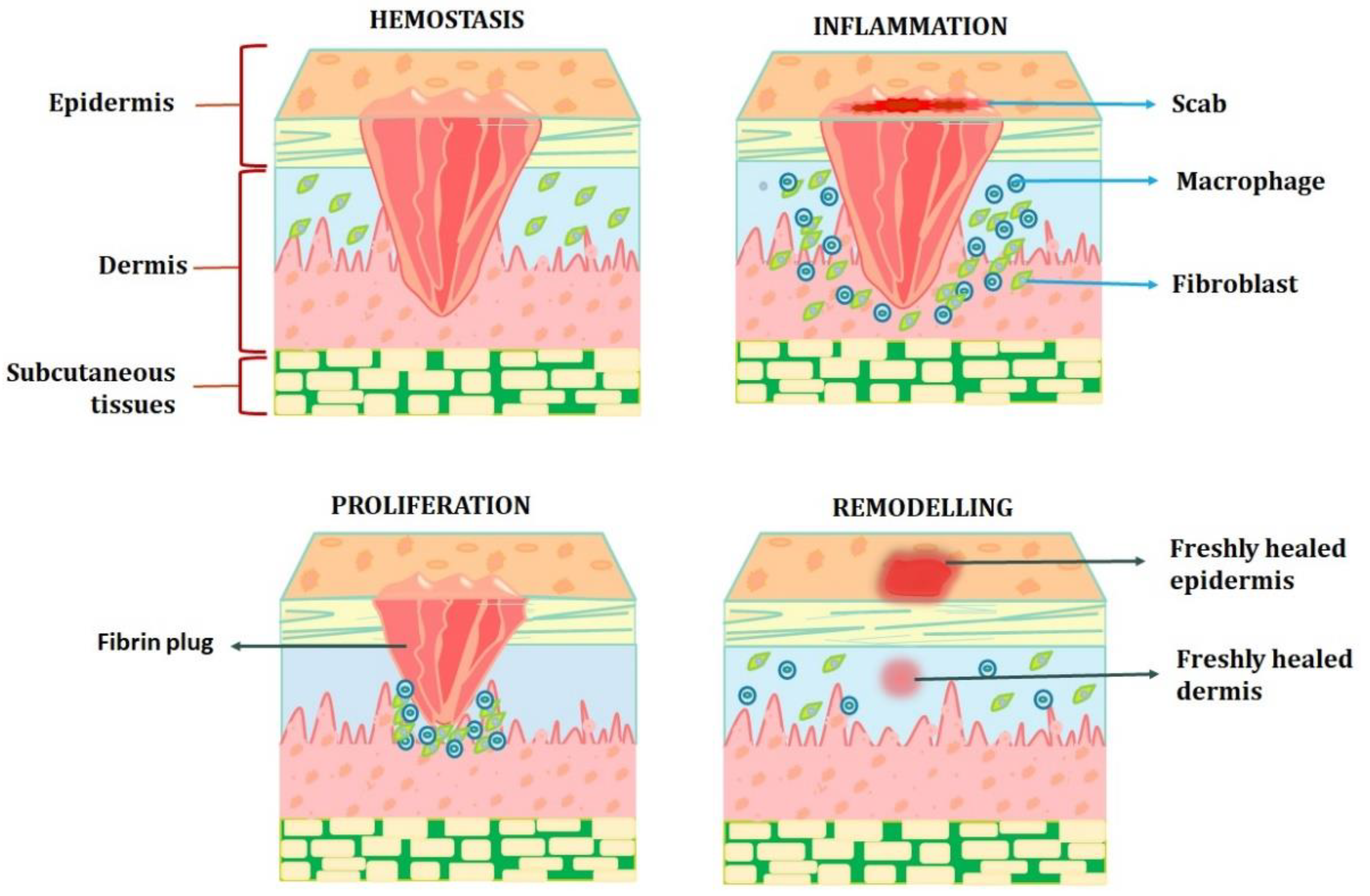
3. Wound Healing Strategies
3.1. Cellular Activity Initiators
DNA synthesis is promoted in fibroblast cells by secretions from healthy wounds. Conversely, the same fibroblasts are inhibited by the secretions from long-lasting, nonhealing wounds, such as leg ulcers. Interestingly, heating the fluid contents of a chronic wound denatures them, removing the inhibitory impact and restoring fibroblast growth. As before, fibroblasts from chronic wounds have the worst response to growth factors than fibroblasts from acute wounds, suggesting that the fibroblasts in chronic wounds are also harmed. The most promising biomarkers are proteases and cytokines. Traditional medicines such as honey, curcumin, and tannin have been studied using modern pharmaceutical practices to learn how they affect cellular activity. One would anticipate that cytokine release, which represents neutrophil and macrophage activity, would increase in an effort to trigger a fibroblast response if fibroblasts stop responding. The pro-inflammatory cytokines IL-1 and TNF-α were found in higher concentrations in non-healing wounds than in healing wounds. When the healing starts, the levels significantly decrease.
Several methods were used to modulate macrophages, including blocking IL-1 or TNF-α, inhibiting the inflammasome pharmacologically, neutralising MCP-1, and chelating iron with desferrioxamine. Sulphated hyaluronic acid is internalised by macrophages after being identified by CD44 and the scavenger receptors CD36 and LOX-1. Most notably, it prevents the phosphorylation of the transcription factors including pNFkB, pSTAT1, and IRF5 that are involved in M1-like activation states and the production of pro-inflammatory genes. Sulphated hyaluronic acid regulates macrophage activation in vivo.
By altering fibroblast activity and proliferation, anti-fibrotic medications such as mitomycin C and 5-FU stop the formation of scars. By disrupting pyrimidine metabolism, the anti-proliferative activity of 5-FU is mediated. By preventing the production of thymidine nucleotides, it prevents DNA synthesis, leading to cell death. It has long-lasting effects on Tenon’s fibroblasts and can effectively limit fibroblast development [12]. By reducing to an alkylating agent, mitomycin C is activated and subsequently works by cross-linking DNA. Mitomycin C can impede not just DNA replication but also mitosis and protein synthesis.
3.2. Collagen Synthesis Activators
3.3. Angiogenesis Activators
3.4. Cytokine and Growth Factor Activators
3.5. Antimicrobials
There is a lot of debate about the application of topical antibiotics to wounds. Topical antimicrobial agents are described as substances that can eliminate, suppress, or lessen the number of bacteria. These substances include disinfectants, antiseptics, and antibiotics. Topical antimicrobial medicines are essential to topical burn care because they are used to prevent and control infection. The ideal topical preventive antimicrobial agent would be able to enter necrotic tissue without being absorbed by the body, have a broad spectrum of activity, a lengthy duration of action, have low toxicity, and have several other qualities [18].
3.6. Stem Cell-Based Therapy
3.7. Herbal Alternatives Acting as Activators for Wound Healing Factors
4. Localised Delivery Systems for Wound Healing
4.1. Microspheres/Microcarriers
4.2. Inorganic Nanoparticles
4.3. Hydrogel
4.4. Vesicles Delivery System
4.5. Emulsifying Drug Delivery System
4.6. Nanofiber/Film/Membrane
A wide range of polymers can be used to create nanofibres. For dressing nanofibres, there are only three types of polymers now available: natural polymers, synthetic polymers, and mixed polymers. Natural polymers are appropriate for use in biomedical applications due to their wide range of benefits, including biocompatibility, non-toxicity, biodegradability, antibacterial properties, and desirable mechanical structure. In the procedure, the solvent evaporates. On the other hand, nanofibres are made of a polymeric base, which makes up most of the fibre’s composition, and a bioactive molecule (such as a protein, hormone, or medication), or another type of polymer, but in a lesser amount than the base polymer. Currently, the three primary techniques for producing nanofibres are electrospinning, the phase-separation method, and the self-assembly method. The method that produces nanofibres most frequently is electrospinning. Depending on the electrospinning technique employed, various types of nanofibres can be produced. Today, the commercialisation of electrospinning equipment is advancing quickly. The most popular electrospinning methods include bubble electrospinning, melt electrospinning, coaxial electrospinning, self-bundling electrospinning, and nano-spider electrospinning [65][66][67][68][69][70][71][72][73][74][75][76][77].
Tissue engineering and wound healing are two of the most important and intriguing biomedical uses of nanofibres. Nanofibres have been utilised in the treatment of diabetic ulcers and wounds to aid in wound healing, haemostasis, skin regeneration, and wound dressing. Nanofibre keeps the wound surface moist while healing because it can hold more moisture in its structure. As a result, the nanofibres cannot adhere to the surface of the wound. Additionally, the porous nanofibre network makes it simpler for oxygen to diffuse into the wound area. To remove toxins from the blood of individuals with kidney failure, wearable blood purification systems may integrate the nanofibre membrane. Nanofibre scaffolds hold great promise for wound healing. These scaffolds are used in the treatment of diabetic ulcers, skin rejuvenation, wound dressings to encourage healing, and haemostasis [78][79].
4.7. Foam Dressings
4.8. Biological Dressings
4.9. Charcoal Dressings
4.10. Three-Dimensional Skin Substitutes
4.11. Dendrimers
4.12. Carbon Nanotubes
4.13. Microneedle Drug Delivery Systems
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020634
References
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239.
- Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072.
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378.
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610.
- Yuan, Z.; Zhang, K.; Jiao, X.; Cheng, Y.; Zhang, Y.; Zhang, P.; Zhang, X.; Wen, Y. A controllable local drug delivery system based on porous fibers for synergistic treatment of melanoma and promoting wound healing. Biomater. Sci. 2019, 7, 5084–5096.
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin. Drug Deliv. 2017, 14, 897–908.
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed. Mater. 2021, 16, 045013.
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108.
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360.
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288.
- Fatehi, P.; Abbasi, M. Medicinal plants used in wound dressings made of electrospun nanofibers. J. Tissue Eng. Regen. Med. 2020, 14, 1527–1548.
- Cabourne, E.; Clarke, J.C.; Schlottmann, P.G.; Evans, J.R. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst. Rev. 2015, 2015, CD006259.
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833.
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211.
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217.
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232.
- Heinrich, P.-C.; Behrmann, I.; Haan, S.; Hermanns, H.-M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 15, 374.
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Schoung, J.Y.; Huang, Y.B.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449.
- Tamahkar, E.; Özkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536.
- Sabitha, M.; Rajiv, S. Preparation and characterization of ampicillin-incorporated electrospun polyurethane scaffolds for wound healing and infection control. Polym. Eng. Sci. 2015, 55, 541–548.
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible amoxicillin-grafted bacterial cellulose sponges for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870.
- Basha, M.; AbouSamra, M.M.; Awad, G.A.; Mansy, S.S. A potential antibacterial wound dressing of cefadroxil chitosan nanoparticles in situ gel: Fabrication, in vitro optimization and in vivo evaluation. Int. J. Pharm. 2018, 544, 129–140.
- Nikdel, M.; Rajabinejad, H.; Yaghoubi, H.; Mikaeiliagah, E.; Cella, M.A.; Sadeghianmaryan, A.; Ahmadi, A. Fabrication of cellulosic nonwoven material coated with polyvinyl alcohol and zinc oxide/mesoporous silica nanoparticles for wound dressing purposes with cephalexin delivery. ECS J. Solid State Sci. Technol. 2021, 10, 057003.
- Rădulescu, M.; Holban, A.-M.; Mogoantă, L.; Bălşeanu, T.-A.; Mogos-anu, G.-D.; Savu, D.; Popescu, R.C.; Fufă, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, Characterization, and Evaluation of Bionanocomposites Based on Natural Polymers and Antibiotics for Wound Healing Applications. Molecules 2016, 21, 761.
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly (vinyl alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 1, 2200201.
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; ÇakırKoç, R.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method. Int. J. Polym. Mater. Polym. Biomater. 2022, 13, 71.
- de Souza, R.F.B.; de Souza, F.C.B.; Moraes, Â.M. Polysaccharide-based membranes loaded with erythromycin for application as wound dressings. Appl. Polym. Sci. 2016, 10, 133.
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 1, 77.
- Khampieng, T.; Wnek, G.-E.; Supaphol, P. Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats:In vitro drug release and antibacterial properties investigation. J. Biomater. Sci. Polym. Ed. 2014, 25, 1292–1305.
- Akota, I.; Alvsaker, B.; Bjørnland, T. The effect of locally applied gauze drain impregnated with chlortetracycline ointment in mandibular third-molar surgery. Acta Odontol. Scand. 1998, 56, 25–29.
- Abbott, P.V.; Hume, W.R.; Pearman, J.W. Antibiotics and endodontics. Aust. Dental. J. 1990, 35, 50–60.
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C 2018, 1, 86.
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels containing antibiofilm and antimicrobial agents beneficial for biofilm-associated wound infection: Formulation characterizations and In vitro study. AAPS PharmSciTech 2018, 19, 1219–1230.
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M.T. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148.
- Nitanan, T.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Neomycin-loaded poly (styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int. J. Pharm. 2013, 1, 448.
- Denkbaş, E.U.R.B.; Öztürk, E.; Özdem&unknownr, N.; Agalar, C. Norfloxacin-loaded chitosan sponges as wound dressing material. J. Biomater. Appl. 2004, 18, 291–303.
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldonic, L.; Bertorelli, R.; Athanassiou, A.; Bayera, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144.
- Li, H.; Williams, G.R.; Wu, J.; Wang, H.; Sun, X.; Zhu, L.M. Poly (N-isopropylacrylamide)/poly (l-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater. Sci. Eng. C 2017, 1, 79.
- Pamfil, D.; Vasile, C.; Tarţău, L.; Vereştiuc, L.; Poiată, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride–modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381.
- Pásztor, N.; Rédai, E.; Szabó, Z.I.; Sipos, E. Preparation and Characterization of Levofloxacin-Loaded Nanofibers as Potential Wound Dressings. Acta Med. Marisiensis 2017, 1, 63.
- Singh, B.; Dhiman, A. Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties. RSC Adv. 2015, 5, 44666–44678.
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 1, 25.
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656.
- Rolston, K.V.I.; Dholakia, N.; Ho, D.H.; LeBlanc, B.; Dvorak, T.; Streeter, H. In-vitro activity of ramoplanin (a novel lipoglycopeptide), vancomycin, and teicoplanin against gram-positive clinical isolates from cancer patients. J. Antimicrob. Chemother. 1996, 38, 265–269.
- Dou, J.L.; Jiang, Y.W.; Xie, J.Q.; Zhang, X.G. New is old, and old is new: Recent advances in antibiotic-based, antibiotic-free and ethnomedical treatments against methicillin-resistant Staphylococcus aureus wound infections. Int. J. Mol. Sci. 2016, 17, 617.
- Fajardo, A.R.; Lopes, L.C.; Caleare, A.O.; Britta, E.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Silver sulfadiazine loaded chitosan/chondroitin sulfate films for a potential wound dressing application. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 588–595.
- Hasselmann, J.; Kühme, T.; Acosta, S. Antibiotic prophylaxis with trimethoprim/sulfamethoxazole instead of cloxacillin fails to improve inguinal surgical site infection rate after vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 129–134.
- Gjorevski, N.; Nikolaev, M.; Brown, T.E.; Mitrofanova, O.; Brandenberg, N.; DelRio, F.W.; Yavitt, F.M.; Liberali, P.; Anseth, K.S.; Lutolf, M.P. Tissue geometry drives deterministic organoid patterning. Science 2022, 375, eaaw9021.
- ValadanTahbaz, S.; Azimi, L.; Asadian, M.; Lari, A.R. Evaluation of synergistic effect of tazobactam with meropenem and ciprofloxacin against multi-drug resistant Acinetobacter baumannii isolated from burn patients in Tehran. GMS Hyg. Infect. Control 2019, 14, Doc08.
- Yang, M.; Hu, Z.; Hu, F. Nosocomial meningitis caused by Acinetobacter baumannii: Risk factors and their impact on patient outcomes and treatments. Future Microbiol. 2012, 7, 787–793.
- NourianDehkordi, A.; MirahmadiBabaheydari, F.; Chehelgerdi, M.; RaeisiDehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111.
- Gonzales, K.A.U.; Fuchs, E. Skin and its regenerative powers: An alliance between stem cells and their niche. Dev. Cell 2017, 43, 387–401.
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617.
- Huang, S.; Lu, G.; Wu, Y.; Jirigala, E.; Xu, Y.; Ma, K.; Fu, X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol. Sci. 2012, 66, 29–36.
- MofazzalJahromi, M.; SahandiZangabad, P.; MoosaviBasri, S.M.; SahandiZangabad, K.; Ghamarypour, A.; Aref, A.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64.
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392.
- Likus, W.; Bajor, G.; Siemianowicz, K. Nanosilver—Does it have only one face? Acta Biochim. Pol. 2013, 60, 495–501.
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.; Hu, X.K. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942.
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656.
- Dhaliwal, K.; Lopez, N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018, 23 (Suppl. 9), S24–S27.
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169.
- Xu, H.L.; Chen, P.P.; ZhuGe, D.L.; Zhu, Q.Y.; Jin, B.H.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6, 1700344.
- Reimer, K.; Vogt, P.M.; Broegmann, B.; Hauser, J.; Rossbach, O.; Kramer, A.; Rudolph, P.; Bosse, B.; Schreier, H.; Fleischer, W. An innovative topical drug formulation for wound healing and infection treatment: In vitro and in vivo investigations of a povidone-iodine liposome hydrogel. Dermatology 2000, 201, 235–241.
- Koshak, A.E.; Algandaby, M.M.; Mujallid, M.I.; Abdel-Naim, A.B.; Alhakamy, N.A.; Fahmy, U.A.; Alfarsi, A.; Badr-Eldin, S.M.; Neamatallah, T.; Nasrullah, M.Z.; et al. Wound Healing Activity of Opuntia ficus-indica Fixed Oil Formulated in a Self-Nanoemulsifying Formulation. Int. J. Nanomed. 2021, 16, 3889–3905.
- Grip, J.; Engstad, R.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280.
- Xu, X.; Wang, X.; Qin, C.; Khan, A.U.R.; Zhang, W.; Mo, X. Silk fibroin/poly-(L-lactide-co-caprolactone) nanofiber scaffolds loaded with Huangbai Liniment to accelerate diabetic wound healing. Colloids Surf. B Biointerfaces 2021, 199, 111557.
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Shahari, M.S.B.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899.
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143.
- Li, Y.; Zhang, Z.Z. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des. Dev. Ther. 2018, 12, 1453–1466.
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a promising nanoplatform for oral delivery of an alkaloid nutraceutical: Improved pharmacokinetic profile and snowballed hypoglycemic effect in diabetic rats. Drug Deliv. 2022, 29, 2694–2704.
- Ternullo, S.; Schulte Werning, L.V.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-in-Deformable Liposomes-in-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2019, 12, 8.
- Cui, M.D.; Pan, Z.H.; Pan, L.Q. DangguiBuxue Extract-Loaded Liposomes in Thermosensitive Gel Enhance In Vivo Dermal Wound Healing via Activation of the VEGF/PI3K/Akt and TGF-β/SmadsSignaling Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8407249.
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale. 2020, 12, 2268–2291.
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91.
- Souriyan-Reyhani pour, H.; Khajavi, R.; Yazdanshenas, M.E.; Zahedi, P.; Mirjalili, M. Cellulose acetate/poly(vinyl alcohol) hybrid fibrous mat containing tetracycline hydrochloride and phenytoin sodium: Morphology, drug release, antibacterial, and cell culture studies. J. Bioact. Compat. Polym. 2018, 33, 597–611.
- Kong, Y.; Xu, R.; Darabi, M.A.; Zhong, W.; Luo, G.; Xing, M.M.Q.; Wu, J. Fast and safe fabrication of a free-standing chitosan/alginate nanomembrane to promote stem cell delivery and wound healing. Int. J. Nanomed. 2016, 11, 2543–2555.
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017, 9, eaai9044.
- Cao, L.; Shao, G.; Ren, F.; Yang, M.; Nie, Y.; Peng, Q.; Zhang, P. Cerium oxide nanoparticle-loaded polyvinyl alcohol nanogels delivery for wound healing care systems on surgery. Drug Deliv. 2021, 28, 390–399.
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747.
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10, CD011332.
- Jones, R.N. Critical assessment of the newer non-quinolone oral antimicrobial agents. Antimicrob. Newsl. 1989, 6, 53–60.
- Sillmon, K.; Moran, C.; Shook, L.; Lawson, C.; Burfield, A.H. The Use of Prophylactic Foam Dressings for Prevention of Hospital-Acquired Pressure Injuries: A Systematic Review. J. Wound Ostomy Cont. Nurs. 2021, 48, 211–218.
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, e2100477.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35.
- Jin, S.; Oh, Y.N.; Son, Y.R.; Kwon, B.; Park, J.H.; Gang, M.J.; Kim, B.W.; Kwon, H.J. Three-Dimensional Skin Tissue Printing with Human Skin Cell Lines and Mouse Skin-Derived Epidermal and Dermal Cells. J Microbiol. Biotechnol. 2022, 32, 238–247.
- Tan, S.H.; Ngo, Z.H.; Sci, D.B.; Leavesley, D.; Liang, K. Recent Advances in the Design of Three-Dimensional and Bioprinted Scaffolds for Full-Thickness Wound Healing. Tissue Eng. Part B Rev. 2022, 28, 160–181.
- Gupta, P.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors 2022, 12, 462.
- Zhang, Y.; Wang, B.; Meng, X.; Sun, G.; Gao, C. Influences of acid-treated multiwalled carbon nanotubes on fibroblasts: Proliferation, adhesion, migration, and wound healing. Ann. Biomed. Eng. 2011, 39, 414–426.
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mousa, A. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. Int. J. Nanomed. 2018, 13, 7195–7206.
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation Behavior In Vitro of Carbon Nanotubes (CNTs)/Poly(lactic acid) (PLA) Composite Suture. Polymers 2019, 11, 1015.
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano 2021, 15, 17842–17853.
- Wang, Y.; Lu, H.; Guo, M.; Chu, J.; Gao, B.; He, B. Personalized and Programmable Microneedle Dressing for Promoting Wound Healing. Adv. Healthc. Mater. 2022, 11, e2101659.
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259.
- Chi, J.; Sun, L.; Cai, L.; Fan, L.; Shao, C.; Shang, L.; Zhao, Y. Chinese herb microneedle patch for wound healing. Bioact. Mater. 2021, 6, 3507–3514.
- Wang, Y.; Gao, B.; He, B. Toward Efficient Wound Management: Bioinspired Microfluidic and Microneedle Patch. Small 2022, 19, e2206270.
