Two-dimensional sp2 hybridized graphene has become a material of choice in research due to the excellent properties it displays electrically, thermally, optically and mechanically. Noble nanomaterials also present special physical and chemical properties and, therefore, they provide model building blocks in modifying nanoscale structures for various applications, ranging from nanomedicine to catalysis and optics. The introduction of noble metal nanoparticles (NPs) (Au, Ag and Pd) into chemically derived graphene is important in opening new avenues for both materials in different fields where they can provide hybrid materials with exceptional performance due to the synergistical result of the specific properties of each of the materials.
2. Methods of Synthesis NPs@GO Nanocomposites
1. Chemical Reduction
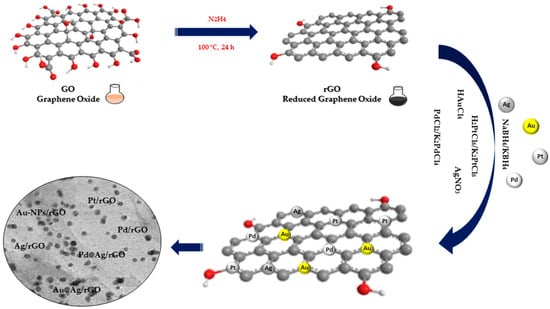
Chemical reduction is the most commonly used method to effectively immobilize NPs on GO and rGO. This method involves noble metal ions in solution being reduced to NPs on GO nanowires through additional reductants such as NaBH
4, ascorbic acid, sodium citrate or hydrazine (
Figure 1). Usually, the GO and rGO dispersion is firstly mixed with noble metal salt solutions, following which the noble metal ions begin adsorbing on the GO and rGO nanosheet surface through electrostatic interaction. Following this, the reducing agents in the mixture reduce the noble metal ions adsorbed in NPs on GO and rGO nanowires [
24].
Figure 1. Chemical reduction synthesis of noble metal nanocomposites.
The three fundamental steps constituting the reduction process are as follows: (1) adsorption/reduction, (2) nucleation and (3) growth. The presence of oxygen-containing functional groups on the surface of the GO and rGO favors the adsorption of free metal ions through electrostatic interactions, followed by the reduction in metal ions by a reducing agent and finally the growth of NPs on the GO and rGO sheets. In spite of the formation of MNPs by chemical reduction being a facile process, this technique is limited due to difficulties sterned from size and morphology of the NPs, which can potentially result in polydisperse and large sizes of on GO and rGO surfaces [
18].
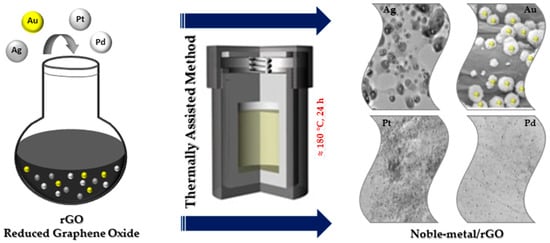
2. Thermally Assisted Method
The thermally assisted method is one of the important methods used to fabricate NPs@GO nanocomposites more simply at high temperature (
Figure 2) [
13]. Thermally assisted synthesis is an easy and efficient method used to immobilize NPs on GO. The speed of the process makes the size and the distribution of the NPs@GO, in this case, difficult to control.
Figure 2. Thermally assisted synthesis of noble metal nanocomposites.
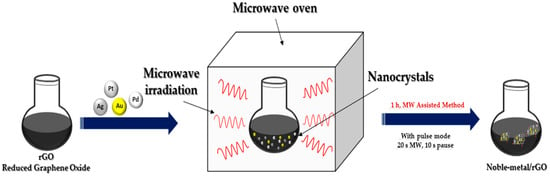
3. Microwave Irradiation Method
In recent years, microwave irradiation has been used as an eco-friendly method in the synthesizing organic, inorganic and inorganic–organic hybrid materials due to its well-known advantages over conventional synthetic methods. The size as well as distribution of NPs synthesized using the light or microwave irradiation method could be easily controlled compared to reductant-assisted or thermal-assisted reduction method, by changing the intensity, power and irradiation time of the light or microwave (
Figure 3). Another important property of microwave irradiation synthesis is that along with the reduction in metals, simultaneous reduction in graphene oxide is possible [
13].
Figure 3. Microwave irradiation synthesis of noble metal nanocomposites.
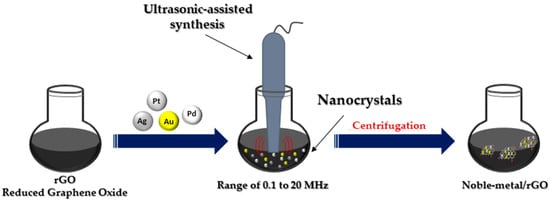
4. Ultrasonication Method
The ultrasonic method (
Figure 4) leads to the rapid heating of the liquid to temperatures of 5000 K in a few nanoseconds, resulting in microbubbles with an effective effect. These microbubbles act as chemical reactors. Oxidative and reducing radicals are generated in the cavitation effect during sonolysis. Sonication in the range of 20 to 1000 kHz leads to the formation of MNPs from metal precursor solution. The collapse of these microbubbles leads to the generation of high temperatures inside the bubbles [
18]. Ultrasonic testing techniques are widely accepted for testing materials in many industries, including power generation, steel, aluminum, titanium production, airframe manufacturing, jet engine manufacturing and shipbuilding [
42].
Figure 4. Ultrasonic-assisted synthesis of noble metal nanocomposites.
3. Advantages and Disadvantages of the Synthesis Methods of Noble Metals Functionalized on Graphene Oxide
In recent years, different methods have been proposed for the synthesis of nanoparticles deposited on a graphene support. The choice of the most suitable method has the greatest importance in terms of the structure and catalytic efficiency of the catalysts. Table 1 presents the advantages, disadvantages and applications of the most known methods used in the synthesis of nanoparticles deposited on a graphene support.
In conclusion, the most valuable method among the preparation methods of graphene-deposited nanomaterial catalysts is microwave field irradiation, especially due to the short synthesis time, the fast and uniform heating and the significant challenge in controlling uniformity of the metal nanoparticle’s decoration on the graphene surface. By applying irradiation in the microwave field, under the influence of temperature, homogeneous reaction centers are formed in the reaction medium at the interface between the irradiation-sensitive graphene support and the metal precursor. Additionally, the presence of a reducing agent in the reaction medium means that the precursor can be converted to its metallic form by microwave irradiation.
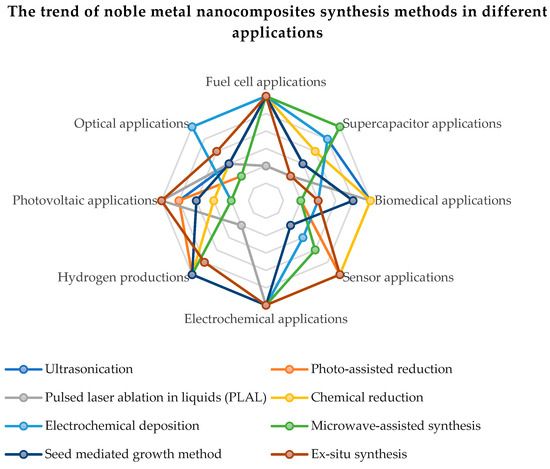
The qualities of noble metals have demonstrated a special efficiency in the electrocatalytic activity and the electrochemical stability of compounds based on carbon and graphene oxide. In order to improve the oxygen reduction reaction (ORR) and the quality of hydrogen adsorption and desorption, a higher electrochemical active surface area (ECSA) of the catalyst based on noble metals is necessary. The intrinsic increase in the active surface is proportional to the metal content in the chemical compound and to the dispersion of metal nanoparticles on the rGO sheets. The uniform distribution and surface morphology of noble metal nanoparticles on rGO have an effect on the ORR. An excessive reaction energy can cause an agglomeration of the noble metal nanoparticles, leading to particle sizes over 10 nm and the suppression of catalytic activity by reducing the active surface. Figure 5 present the trend of noble metal nanocomposites synthesis methods in different applications. Thus, it can be seen that the most applications of graphene functionalized with noble metals are in applications with fuel cells, renewable energy sources (photovoltaics, production of green hydrogen) and supercapacitors.
Figure 5. The trend of noble metal nanocomposites synthesis methods in different applications.
This entry is adapted from the peer-reviewed paper 10.3390/nano13040783