p300 acts as a transcription coactivator and an acetyltransferase that plays an important role in tumourigenesis and progression. In previous studies, it has been confirmed that p300 is an important regulator in regulating the evolution of malignant tumours and it also has extensive functions. From the perspective of non-posttranslational modification, it has been proven that p300 can participate in regulating many pathophysiological processes, such as activating oncogene transcription, promoting tumour cell growth, inducing apoptosis, regulating immune function and affecting embryo development. p300 has been found to act as an acetyltransferase that catalyses a variety of protein modification types, such as acetylation, propanylation, butyylation, 2-hydroxyisobutyration, and lactylation. Under the catalysis of this acetyltransferase, it plays its crucial tumourigenic driving role in many malignant tumours. Therefore, the function of p300 acetyltransferase has gradually become a research hotspot. From a posttranslational modification perspective, p300 is involved in the activation of multiple transcription factors and additional processes that promote malignant biological behaviours, such as tumour cell proliferation, migration, and invasion, as well as tumour cell apoptosis, drug resistance, and metabolism.
- p300
- tumours
- inhibitor
- posttranslational modification
- Acetyltransferase
1. p300 Can Regulate Transcription
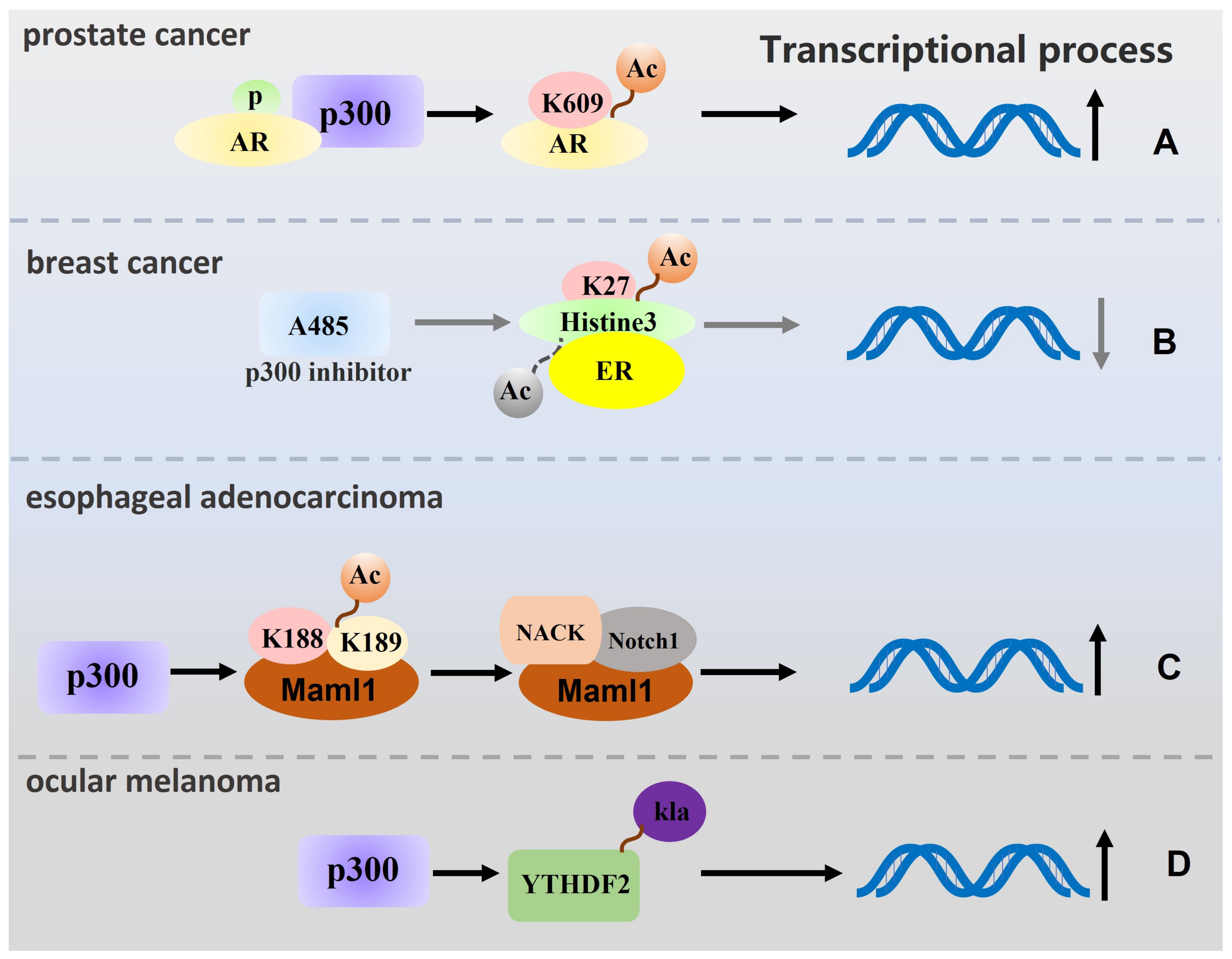
2. p300 Regulates Tumour Cell Proliferation, Migration, and Invasion
3. p300 Regulates Tumour Cell Apoptosis
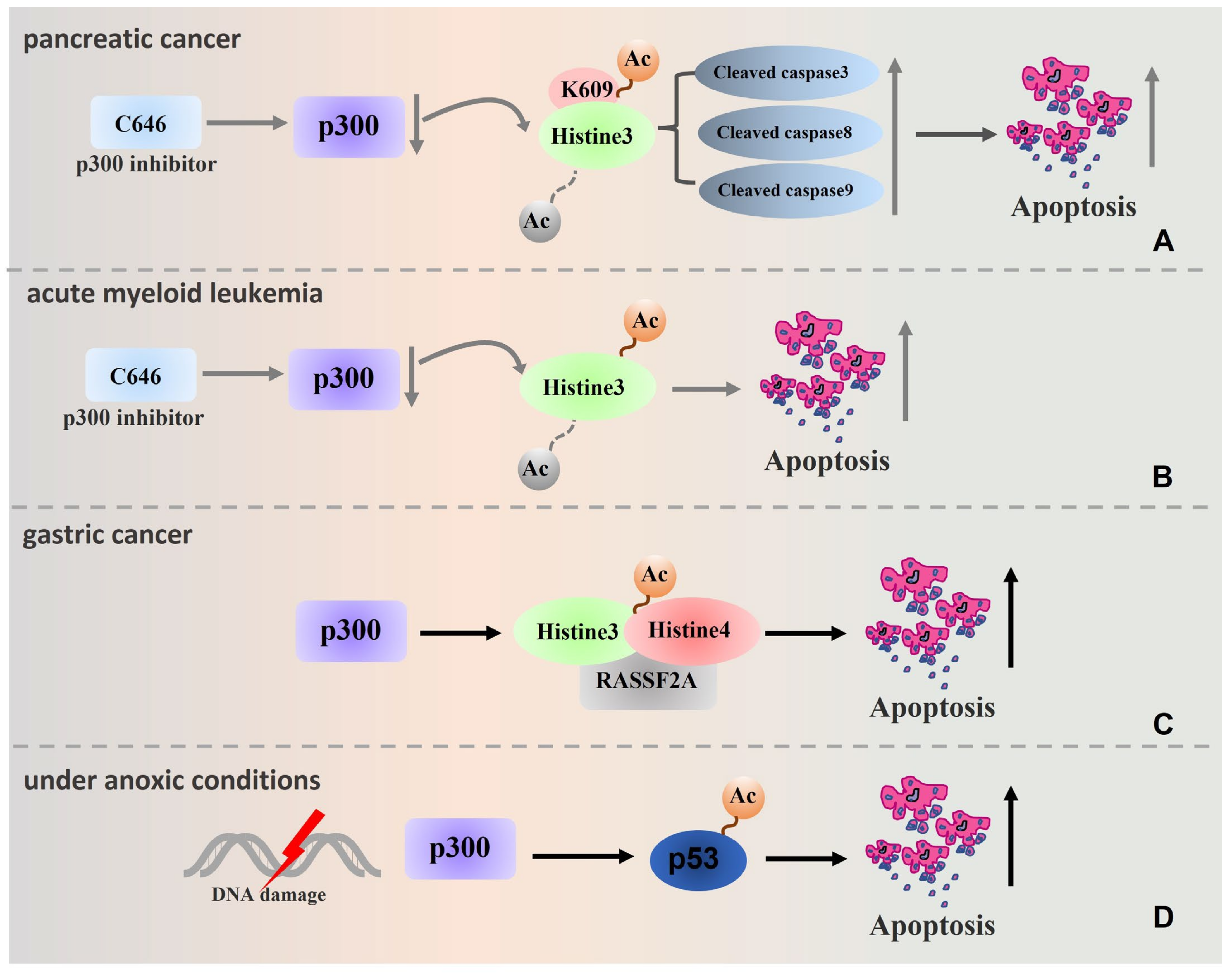
4. p300 Regulates the Formation of Tumour Drug Resistance
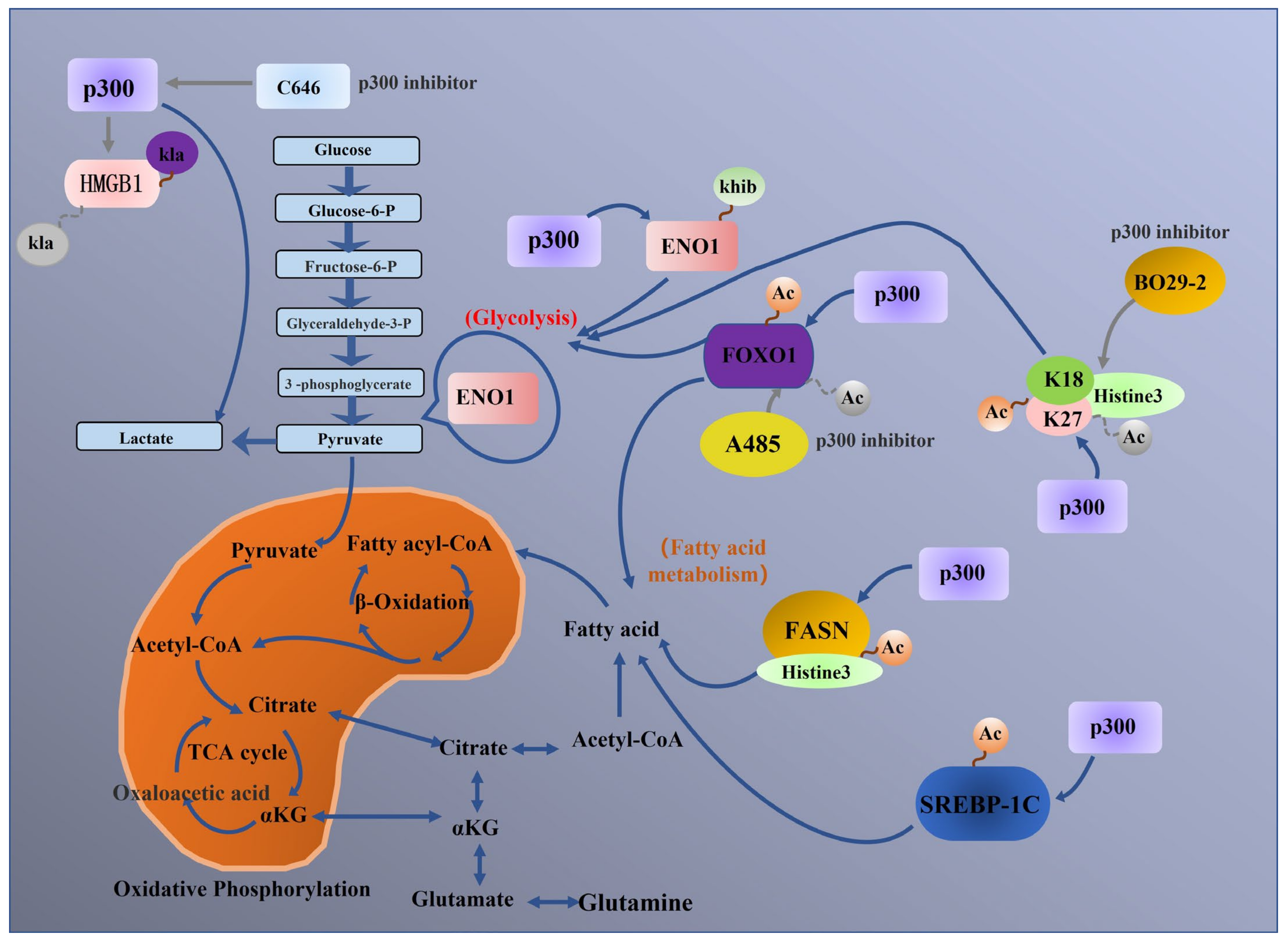
5. p300 Regulates Tumour Metabolic Processes
This entry is adapted from the peer-reviewed paper 10.3390/biom13030417
References
- Liu, C.; Pan, Y.; Wang, X.; Lu, J.; Huang, B.; Li, X. Activation of RASSF2A by p300 induces late apoptosis through histone hyperacetylation. Cell Biol. Int. 2010, 34, 1133–1139.
- Bandyopadhyay, D.; Okan, N.A.; Bales, E.; Nascimento, L.; Cole, P.A.; Medrano, E.E. Down-regulation of p300/CBP histone acetyltransferase activates a senescence checkpoint in human melanocytes. Cancer Res. 2002, 62, 6231–6239.
- Haery, L.; Lugo-Picó, J.G.; Henry, R.A.; Andrews, A.J.; Gilmore, T.D. Histone acetyltransferase-deficient p300 mutants in diffuse large B cell lymphoma have altered transcriptional regulatory activities and are required for optimal cell growth. Mol. Cancer 2014, 13, 1–13.
- Sawant, M.; Mahajan, K.; Renganathan, A.; Weimholt, C.; Luo, J.; Kukshal, V.; Jez, J.M.; Jeon, M.S.; Zhang, B.; Li, T.; et al. Chronologically modified androgen receptor in recurrent castration-resistant prostate cancer and its therapeutic targeting. Sci. Transl. Med. 2022, 14, eabg4132.
- Ianculescu, I.; Wu, D.Y.; Siegmund, K.D.; Stallcup, M.R. Selective roles for cAMP response element-binding protein binding protein and p300 protein as coregulators for androgen-regulated gene expression in advanced prostate cancer cells. J. Biol. Chem. 2012, 287, 4000–4013.
- Waddell, A.; Mahmud, I.; Ding, H.; Huo, Z.; Liao, D. Pharmacological Inhibition of CBP/p300 Blocks Estrogen Receptor Alpha (ERalpha) Function through Suppressing Enhancer H3K27 Acetylation in Luminal Breast Cancer. Cancers 2021, 13, 2799.
- Cho, M.H.; Park, J.H.; Choi, H.J.; Park, M.K.; Won, H.Y.; Park, Y.J.; Lee, C.H.; Oh, S.H.; Song, Y.S.; Kim, H.S.; et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat. Commun 2015, 6, 7821.
- Jin, K.; Zhou, W.; Han, X.; Wang, Z.; Li, B.; Jeffries, S.; Tao, W.; Robbins, D.J.; Capobianco, A.J. Acetylation of Mastermind-like 1 by p300 Drives the Recruitment of NACK to Initiate Notch-Dependent Transcription. Cancer Res. 2017, 77, 4228–4237.
- He, H.; Wang, D.; Yao, H.; Wei, Z.; Lai, Y.; Hu, J.; Liu, X.; Wang, Y.; Zhou, H.; Wang, N.; et al. Transcriptional factors p300 and MRTF-A synergistically enhance the expression of migration-related genes in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2015, 467, 813–820.
- Wang, W.; Chen, Y.; Du, R.; Xia, X.; Zhang, Y.; Guo, H.; Ma, J.; Tian, J.; Wang, S. C/EBPbeta enhances immunosuppression activity of myeloid-derived suppressor cells by a P300-mediated acetylation modification. Inflamm. Res. 2022, 71, 1547–1557.
- Hogg, S.J.; Motorna, O.; Cluse, L.A.; Johanson, T.M.; Coughlan, H.D.; Raviram, R.; Myers, R.M.; Costacurta, M.; Todorovski, I.; Pijpers, L.; et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol. Cell 2021, 81, 2183–2200.e2113.
- Ishihama, K.; Yamakawa, M.; Semba, S.; Takeda, H.; Kawata, S.; Kimura, S.; Kimura, W. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J. Clin. Pathol. 2007, 60, 1205–1210.
- Liu, J.; He, D.; Cheng, L.; Huang, C.; Zhang, Y.; Rao, X.; Kong, Y.; Li, C.; Zhang, Z.; Liu, J.; et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene 2020, 39, 3939–3951.
- Cai, L.Y.; Chen, S.J.; Xiao, S.H.; Sun, Q.J.; Ding, C.H.; Zheng, B.N.; Zhu, X.Y.; Liu, S.Q.; Yang, F.; Yang, Y.X.; et al. Targeting p300/CBP Attenuates Hepatocellular Carcinoma Progression through Epigenetic Regulation of Metabolism. Cancer Res. 2021, 81, 860–872.
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 2022, 8, 1–15.
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal. Transduct. Target. Ther. 2022, 7, 305.
- Xiao, X.S.; Cai, M.Y.; Chen, J.W.; Guan, X.Y.; Kung, H.F.; Zeng, Y.X.; Xie, D. High Expression of p300 in Human Breast Cancer Correlates with Tumor Recurrence and Predicts Adverse Prognosis. Chin. J. Cancer Res. 2011, 23, 201–207.
- Yokomizo, C.; Yamaguchi, K.; Itoh, Y.; Nishimura, T.; Umemura, A.; Minami, M.; Yasui, K.; Mitsuyoshi, H.; Fujii, H.; Tochiki, N.; et al. High expression of p300 in HCC predicts shortened overall survival in association with enhanced epithelial mesenchymal transition of HCC cells. Cancer Lett. 2011, 310, 140–147.
- Li, Y.; Yang, H.-X.; Luo, R.-Z.; Zhang, Y.; Li, M.; Wang, X.; Jia, W.-H. High Expression of p300 Has an Unfavorable Impact on Survival in Resectable Esophageal Squamous Cell Carcinoma. Ann. Thorac. Surg. 2011, 91, 1531–1538.
- Chen, M.K.; Cai, M.Y.; Luo, R.Z.; Tian, X.; Liao, Q.M.; Zhang, X.Y.; Han, J.D. Overexpression of p300 correlates with poor prognosis in patients with cutaneous squamous cell carcinoma. Br. J. Derm. 2015, 172, 111–119.
- Attar, N.; Kurdistani, S.K. Exploitation of EP300 and CREBBP Lysine Acetyltransferases by Cancer. Cold. Spring Harb. Perspect. Med. 2017, 7, a026534.
- Manuyakorn, A.; Paulus, R.; Farrell, J.; Dawson, N.A.; Tze, S.; Cheung-Lau, G.; Hines, O.J.; Reber, H.; Seligson, D.B.; Horvath, S.; et al. Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: Results from RTOG 9704. J. Clin. Oncol. 2010, 28, 1358–1365.
- Seligson, D.B.; Horvath, S.; Shi, T.; Yu, H.; Tze, S.; Grunstein, M.; Kurdistani, S.K. Global histone modification patterns predict risk of prostate cancer recurrence. Nature 2005, 435, 1262–1266.
- Mosashvilli, D.; Kahl, P.; Mertens, C.; Holzapfel, S.; Rogenhofer, S.; Hauser, S.; Buttner, R.; Von Ruecker, A.; Muller, S.C.; Ellinger, J. Global histone acetylation levels: Prognostic relevance in patients with renal cell carcinoma. Cancer Sci. 2010, 101, 2664–2669.
- Seligson, D.B.; Horvath, S.; McBrian, M.A.; Mah, V.; Yu, H.; Tze, S.; Wang, Q.; Chia, D.; Goodglick, L.; Kurdistani, S.K. Global levels of histone modifications predict prognosis in different cancers. Am. J. Pathol. 2009, 174, 1619–1628.
- Wang, Z.; Yang, X.; Liu, C.; Li, X.; Zhang, B.; Wang, B.; Zhang, Y.; Song, C.; Zhang, T.; Liu, M.; et al. Acetylation of PHF5A Modulates Stress Responses and Colorectal Carcinogenesis through Alternative Splicing-Mediated Upregulation of KDM3A. Mol. Cell 2019, 74, 1250–1263.e1256.
- Zhou, J.; Zhan, S.; Tan, W.; Cheng, R.; Gong, H.; Zhu, Q. P300 binds to and acetylates MTA2 to promote colorectal cancer cells growth. Biochem. Biophys. Res. Commun. 2014, 444, 387–390.
- Lazarova, D.L.; Wong, T.; Chiaro, C.; Drago, E.; Bordonaro, M. p300 Influences Butyrate-Mediated WNT Hyperactivation In Colorectal Cancer Cells. J. Cancer 2013, 4, 491–501.
- Han, X.; Xiang, X.; Yang, H.; Zhang, H.; Liang, S.; Wei, J.; Yu, J. p300-Catalyzed Lysine Crotonylation Promotes the Proliferation, Invasion, and Migration of HeLa Cells via Heterogeneous Nuclear Ribonucleoprotein A1. Anal. Cell. Pathol. 2020, 2020, 5632342.
- Liao, Z.W.; Zhao, L.; Cai, M.Y.; Xi, M.; He, L.R.; Yu, F.; Zhou, T.C.; Liu, M.Z. P300 promotes migration, invasion and epithelial-mesenchymal transition in a nasopharyngeal carcinoma cell line. Oncol. Lett. 2017, 13, 763–769.
- Zhong, J.; Ding, L.; Bohrer, L.R.; Pan, Y.; Liu, P.; Zhang, J.; Sebo, T.J.; Karnes, R.J.; Tindall, D.J.; van Deursen, J.; et al. p300 acetyltransferase regulates androgen receptor degradation and PTEN-deficient prostate tumorigenesis. Cancer Res. 2014, 74, 1870–1880.
- Wang, Y.; Sun, B.; Zhang, Q.; Dong, H.; Zhang, J. p300 Acetylates JHDM1A to inhibit osteosarcoma carcinogenesis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2891–2899.
- Gao, X.N.; Lin, J.; Ning, Q.Y.; Gao, L.; Yao, Y.S.; Zhou, J.H.; Li, Y.H.; Wang, L.L.; Yu, L. A histone acetyltransferase p300 inhibitor C646 induces cell cycle arrest and apoptosis selectively in AML1-ETO-positive AML cells. PLoS ONE 2013, 8, e55481.
- Dutta, R.; Tiu, B.; Sakamoto, K.M. CBP/p300 acetyltransferase activity in hematologic malignancies. Mol. Genet. Metab. 2016, 119, 37–43.
- Palermo, R.; Checquolo, S.; Giovenco, A.; Grazioli, P.; Kumar, V.; Campese, A.F.; Giorgi, A.; Napolitano, M.; Canettieri, G.; Ferrara, G.; et al. Acetylation controls Notch3 stability and function in T-cell leukemia. Oncogene 2012, 31, 3807–3817.
- Garcia-Carpizo, V.; Ruiz-Llorente, S.; Sarmentero, J.; Grana-Castro, O.; Pisano, D.G.; Barrero, M.J. CREBBP/EP300 bromodomains are critical to sustain the GATA1/MYC regulatory axis in proliferation. Epigenetics Chromatin 2018, 11, 30.
- Ji, C.; Xu, W.; Ding, H.; Chen, Z.; Shi, C.; Han, J.; Yu, L.; Qiao, N.; Zhang, Y.; Cao, X.; et al. The p300 Inhibitor A-485 Exerts Antitumor Activity in Growth Hormone Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2022, 107, e2291–e2300.
- Chen, Q.; Yang, B.; Liu, X.; Zhang, X.D.; Zhang, L.; Liu, T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics 2022, 12, 4935–4948.
- Stimson, L.; Rowlands, M.G.; Newbatt, Y.M.; Smith, N.F.; Raynaud, F.I.; Rogers, P.; Bavetsias, V.; Gorsuch, S.; Jarman, M.; Bannister, A.; et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther. 2005, 4, 1521–1532.
- Guo, L.; Li, H.; Fan, T.; Ma, Y.; Wang, L. Synergistic efficacy of curcumin and anti-programmed cell death-1 in hepatocellular carcinoma. Life Sci. 2021, 279, 119359.
- Yang, H.; Pinello, C.E.; Luo, J.; Li, D.; Wang, Y.; Zhao, L.Y.; Jahn, S.C.; Saldanha, S.A.; Chase, P.; Planck, J.; et al. Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents. Mol. Cancer Ther. 2013, 12, 610–620.
- Ogiwara, H.; Sasaki, M.; Mitachi, T.; Oike, T.; Higuchi, S.; Tominaga, Y.; Kohno, T. Targeting p300 Addiction in CBP-Deficient Cancers Causes Synthetic Lethality by Apoptotic Cell Death due to Abrogation of MYC Expression. Cancer Discov. 2016, 6, 430–445.
- Wang, Y.M.; Gu, M.L.; Meng, F.S.; Jiao, W.R.; Zhou, X.X.; Yao, H.P.; Ji, F. Histone acetyltransferase p300/CBP inhibitor C646 blocks the survival and invasion pathways of gastric cancer cell lines. Int. J. Oncol. 2017, 51, 1860–1868.
- Zhang, B.; Chen, D.; Liu, B.; Dekker, F.J.; Quax, W.J. A novel histone acetyltransferase inhibitor A485 improves sensitivity of non-small-cell lung carcinoma cells to TRAIL. Biochem. Pharm. 2020, 175, 113914.
- Dietze, E.C.; Caldwell, L.E.; Marcom, K.; Collins, S.J.; Yee, L.; Swisshelm, K.; Hobbs, K.B.; Bean, G.R.; Seewaldt, V.L. Retinoids and retinoic acid receptors regulate growth arrest and apoptosis in human mammary epithelial cells and modulate expression of CBP/p300. Microsc. Res. Tech. 2002, 59, 23–40.
- Sharma, V.K.; Lahiri, M. Interplay between p300 and HDAC1 regulate acetylation and stability of Api5 to regulate cell proliferation. Sci. Rep. 2021, 11, 16427.
- Avantaggiati, M.L.; Ogryzko, V.; Gardner, K.; Giordano, A.; Levine, A.S.; Kelly, K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 1997, 89, 1175–1184.
- Ono, H.; Basson, M.D.; Ito, H. P300 inhibition enhances gemcitabine-induced apoptosis of pancreatic cancer. Oncotarget 2016, 7, 51301–51310.
- Fu, L.; Huang, W.; Jing, Y.; Jiang, M.; Zhao, Y.; Shi, J.; Huang, S.; Xue, X.; Zhang, Q.; Tang, J.; et al. AML1-ETO triggers epigenetic activation of early growth response gene l, inducing apoptosis in t(8;21) acute myeloid leukemia. FEBS J. 2014, 281, 1123–1131.
- Janknecht, R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol. Histopathol. 2002, 17, 657–668.
- Gopalakrishna Iyer, N.; Chin, S.F.; Ozdag, H.; Daigo, Y.; Hu, D.E.; Cariati, M.; Brindle, K.; Aparicio, S.; Caldas, C. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc. Natl. Acad. Sci. USA 2004, 101, 7386–7391.
- Mahmud, Z.; Gomes, A.R.; Lee, H.J.; Aimjongjun, S.; Jiramongkol, Y.; Yao, S.; Zona, S.; Alasiri, G.; Gong, G.; Yague, E.; et al. EP300 and SIRT1/6 Co-Regulate Lapatinib Sensitivity Via Modulating FOXO3-Acetylation and Activity in Breast Cancer. Cancers 2019, 11, 1067.
- Shiota, M.; Yokomizo, A.; Kashiwagi, E.; Tada, Y.; Inokuchi, J.; Tatsugami, K.; Kuroiwa, K.; Uchiumi, T.; Seki, N.; Naito, S. Foxo3a expression and acetylation regulate cancer cell growth and sensitivity to cisplatin. Cancer Sci. 2010, 101, 1177–1185.
- Mladek, A.C.; Yan, H.; Tian, S.; Decker, P.A.; Burgenske, D.M.; Bakken, K.; Hu, Z.; He, L.; Connors, M.A.; Carlson, B.L.; et al. RBBP4-p300 axis modulates expression of genes essential for cell survival and is a potential target for therapy in glioblastoma. Neuro. Oncol. 2022, 24, 1261–1272.
- Huang, X.; Yan, J.; Zhang, M.; Wang, Y.; Chen, Y.; Fu, X.; Wei, R.; Zheng, X.L.; Liu, Z.; Zhang, X.; et al. Targeting Epigenetic Crosstalk as a Therapeutic Strategy for EZH2-Aberrant Solid Tumors. Cell 2018, 175, 186–199.e119.
- Gang, X.; Yang, Y.; Zhong, J.; Jiang, K.; Pan, Y.; Jeffrey Karnes, R.; Zhang, J.; Xu, W.; Wang, G.; Huang, H. P300 acetyltransferase regulates fatty acid synthase expression, lipid metabolism and prostate cancer growth. Oncotarget 2016, 7, 15135–15149.
- Ponugoti, B.; Kim, D.H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.Y.; Chiang, C.M.; Veenstra, T.D.; Kemper, J.K. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010, 285, 33959–33970.
- Zhou, F.; Liu, Q.; Zhang, L.; Zhu, Q.; Wang, S.; Zhu, K.; Deng, R.; Liu, Y.; Yuan, G.; Wang, X.; et al. Selective inhibition of CBP/p300 HAT by A-485 results in suppression of lipogenesis and hepatic gluconeogenesis. Cell Death Dis. 2020, 11, 745.
- Huang, H.; Tang, S.; Ji, M.; Tang, Z.; Shimada, M.; Liu, X.; Qi, S.; Locasale, J.W.; Roeder, R.G.; Zhao, Y.; et al. p300-Mediated Lysine 2-Hydroxyisobutyrylation Regulates Glycolysis. Mol. Cell 2018, 70, 663–678.e6.
- Yuan, Y.; Yuan, H.F.; Geng, Y.; Zhao, L.N.; Yun, H.L.; Wang, Y.F.; Yang, G.; Zhang, X.D. Aspirin modulates 2-hydroxyisobutyrylation of ENO1K281 to attenuate the glycolysis and proliferation of hepatoma cells. Biochem. Biophys. Res. Commun. 2021, 560, 172–178.
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146.
- Bundy, J.G.; Iyer, N.G.; Gentile, M.S.; Hu, D.E.; Kettunen, M.; Maia, A.T.; Thorne, N.P.; Brenton, J.D.; Caldas, C.; Brindle, K.M. Metabolic consequences of p300 gene deletion in human colon cancer cells. Cancer Res. 2006, 66, 7606–7614.
