Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
Although psoriasis remains one of the most devastating inflammatory disorders due to its huge negative impact on patients’ quality of life, new “green” treatment approaches still need to be fully explored.
- psoriasis
- essential natural oils
- botanical remedies
- nanotechnology-based treatment
1. Introduction
Psoriasis is a common, yet debilitating, immune-mediated inflammatory condition. It usually appears in the form of reddened, raised lesions or “plaques” on the skin, which may be covered with silver or white-colored scales. Individuals at risk of developing psoriasis often have genetic polymorphisms affecting genes that are involved in the adaptive and innate immune systems and/or skin barrier regulation. Both environmental and genetic factors result in chronic inflammation and growth of psoriatic plaques from hyper-proliferating skin cells [1].
Some medications such as lithium, beta-blockers and antimalarial treatments may trigger psoriasis, as they affect normal cell proliferation and differentiation [2]. Additionally, several environmental factors contribute to psoriasis initiation and exacerbation, including physical trauma and pharyngeal infections (streptococcal throat infections). Smoking, alcohol consumption and obesity have also been linked to the disease incidence and have been implicated in worsening of the patient’s condition, but the basis of a clear relationship between them and psoriasis does not exist [3]. Ethnicity can also affect prevalence of psoriasis, such that around 1–3% of the European population suffer from psoriasis, with prevalence varying depending on the geographical area or ethnic group studied [4]. According to the Global Psoriasis Atlas, within Great Britain, prevalence of psoriasis appears to be on the rise, from 2.3% in 1999 to 2.8% in 2013 [5], while its incidence is lower in Western Europe (1.92%) and in North Africa and the Middle East (0.57% of the total population).
Psoriasis can occur in any stage of life. However, epidemiological evidence suggests that onset occurs at two peak ages. In the UK, approximately 75% of patients have hereditary early onset psoriasis (EOP) before 16 years of age, triggered by HLA-Cw*0602 positive (Type I), which is a very aggressive type, while the remaining 25% have uninherited late onset psoriasis (LOP) after 16 years of age, which is triggered by HLA-Cw*0602 negative (type II) [6]. Psoriasis is considered a multi-factorial disease caused by hyperproliferation of keratinocytes, angiogenesis and abnormal imbalanced cells differentiation, and increased secretions of excessive pro-inflammatory mediators such as interleukins, endothelin and vascular endothelial growth factors [7]. Topical treatment of psoriasis represents the first-line treatment. However, long-term therapy induces several side effects, either local or systemic. Nanotechnology-based drug delivery systems offer a solution to overcome the limitations of conventional therapies. They have different physicochemical characters from their active constituents, such as smaller particle size or different nanoscale materials, enabling deeper penetration and localized accumulation in targeted skin layers in a controlled release manner. For example, some advanced formulations contain surfactants or permeation enhancer components so they have the ability to change the molecular structure and barrier functions of skin and make pores in tight junctions, allowing active constituents to reach deeper skin layers and improve the therapeutic outcome [8].
2. Pathogenesis of Psoriasis
Psoriasis is now known to be driven by a cluster of differentiation cells (CD 4+) and T lymphocyte helper 17 (CD4+ Th17) subset, as well as Th1 cells [9]. Th17 cells produce the cytokine interleukin 17 (IL-17) in response to the cytokine IL-23 [10]. CD8 releases several inflammatory cytokines in the skin, including IL-17, IFN-γ, IL-22 and IL-13; of these, IFN-γ is thought to promote hyperproliferation of keratinocytes in the epidermis, which results in skin thickening [11].
Meanwhile, IL-17 causes abnormal differentiation of keratinocytes and stimulates pro-inflammatory cytokines production such as IL-6 and IL-8. The increased level of vascular endothelial growth factor (VEGF) contributes to angiogenesis, dilatation, and formation of high endothelial venues, reflected as skin redness and erythema, a hallmark in psoriatic lesions. IL-1 mediates the production of IL-2 and IFN-γ by T-cells. It is also responsible for the activation of neutrophils, monocytes, eosinophils and basophils, and stimulates macrophages to synthesize tumor necrosis factor-α (TNF), IL-6 and IL-8. IL-2 triggers B cell differentiation as well as production and action of natural killer (NK) cells, monocytes and macrophages. IL-2 encourages synthesis of IFN-g, TNF, IL-6 and IL-2R, and participates in its self-production. Augmentation in blood vessels number is mediated by IL-8, which also stimulates chemotaxis and neutrophil activation. IL-15 regulates the activation, proliferation and endurance of NK cells. This interleukin also stimulates formation of new blood vessels and T cells expression of IL-17 [12].
To sum up, T cells have a potential role in the development of psoriasis and understanding their role helps to localize the pathogenesis of disease. Th1, Th17, Treg and Th22 cells, and newly identified ‘professional’ IL-17-producing dermal CD T cells, all have significant roles in psoriasis pathogenesis. Environmental factors and pathogens activate the dendritic cells (DCs) and macrophages to release IL-23, IL-1b and other pro-inflammatory cytokines, which in turn activate the innate immunity that manifests as dermal CD T cells that produce IL17, which consecutively stimulates conventional acquired immune responses. IL-17, IL-22 and TNF-α stimulate progressively the process of keratinocytes hyperproliferation and create the inflammatory environment of disease [13]. A diagrammatic representation of the pathogenesis of psoriasis is illustrated in Figure 1.
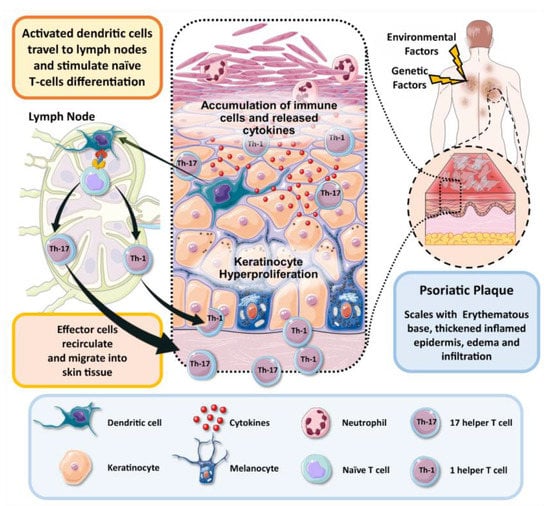
Figure 1. Schematic illustration of pathogenesis of psoriasis.
3. Phenotypes of Psoriasis
One of the main subtypes of psoriasis that accounts for 90% of all cases is Psoriasis Vulgaris (PV). It is chronic plaque psoriasis (CPP) [14], clinically represented as raised itchy or painful thick red patches on the skin which are clearly defined from the non-involved skin surrounding them. These hyperproliferating patches may be covered by silver-white scales, caused by a build-up of keratinocytes, with vascular alteration that participates in enlargement of these psoriatic plaques asymmetrically. Plaques in CPP commonly appear on the outer surfaces of the knees and elbows [15].
Nail psoriasis is also one of the most prevalent types [16]. It is most commonly manifested as nail pitting, which appears as small circular areas underneath the plate, red or white in color. Other symptoms include loosening of the nail from its bed (onycholysis), discoloration due to psoriatic lesions in the nail bed (oil drop lesions), raising of the nail bed (subungual hyperkeratosis), transverse ridges (Beau’s lines), and longitudinal ridges with splitting (onychorrhexis) [17].
Another type is guttate psoriasis, an acute condition prevailing in the youth. Its name is derived from the Latin word “gutta” meaning “droplet”, which refers to the small round pink papules that often appear on the face, ears, and scalp. It is often thought to be triggered by a prior pharyngeal infection or tonsillitis [18].
On the other hand, psoriatic arthritis (PsA) affects approximately 6% of psoriasis patients, with a prevalence of 0.06–0.25% in the United States, 0.21% in Sweden, 0.05% in Turkey and 0.07% in Asia [19]. PsA is similar to rheumatoid arthritis, gout and reactive arthritis since it often manifests as painful inflammation in the joints and tendons, which appears shortly after the development of cutaneous psoriasis. On a cellular level, PsA is thought to be due to accumulation of particular interleukins and other inflammatory mediators in the synovial fluid [20].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15030750
This entry is offline, you can click here to edit this entry!
