SARS-CoV-2 is an enveloped RNA virus with a single-stranded, positive-sense genome of ~29.9 kB in size. The virus consists of four major structural proteins, named spike (S), nucleocapsid (N), envelope (E), and membrane proteins (M). The S protein which is present as a crown-like spike on the outer surface of the virus plays a major role in viral entry into mammalian cells. Specifically, the virus uses the receptor binding domain (RBD) on the S protein to interact with human angiotensin-converting enzyme 2 (ACE2) receptor as a critical initial step to enter target cells. Plants have provided a promising production platform for both bioactive chemical compounds (small molecules) and recombinant therapeutics (big molecules). Plants naturally produce a diverse range of bioactive compounds as secondary metabolites, such as alkaloids, terpenoids/terpenes and polyphenols, which are a rich source of countless antiviral compounds. Plants can also be genetically engineered to produce valuable recombinant therapeutics. This molecular farming in plants has an unprecedented opportunity for developing vaccines, antibodies, and other biologics for pandemic diseases because of its potential advantages, such as low cost, safety, and high production volume.
- coronavirus
- COVID-19
- vaccines
- plant
1. Introduction
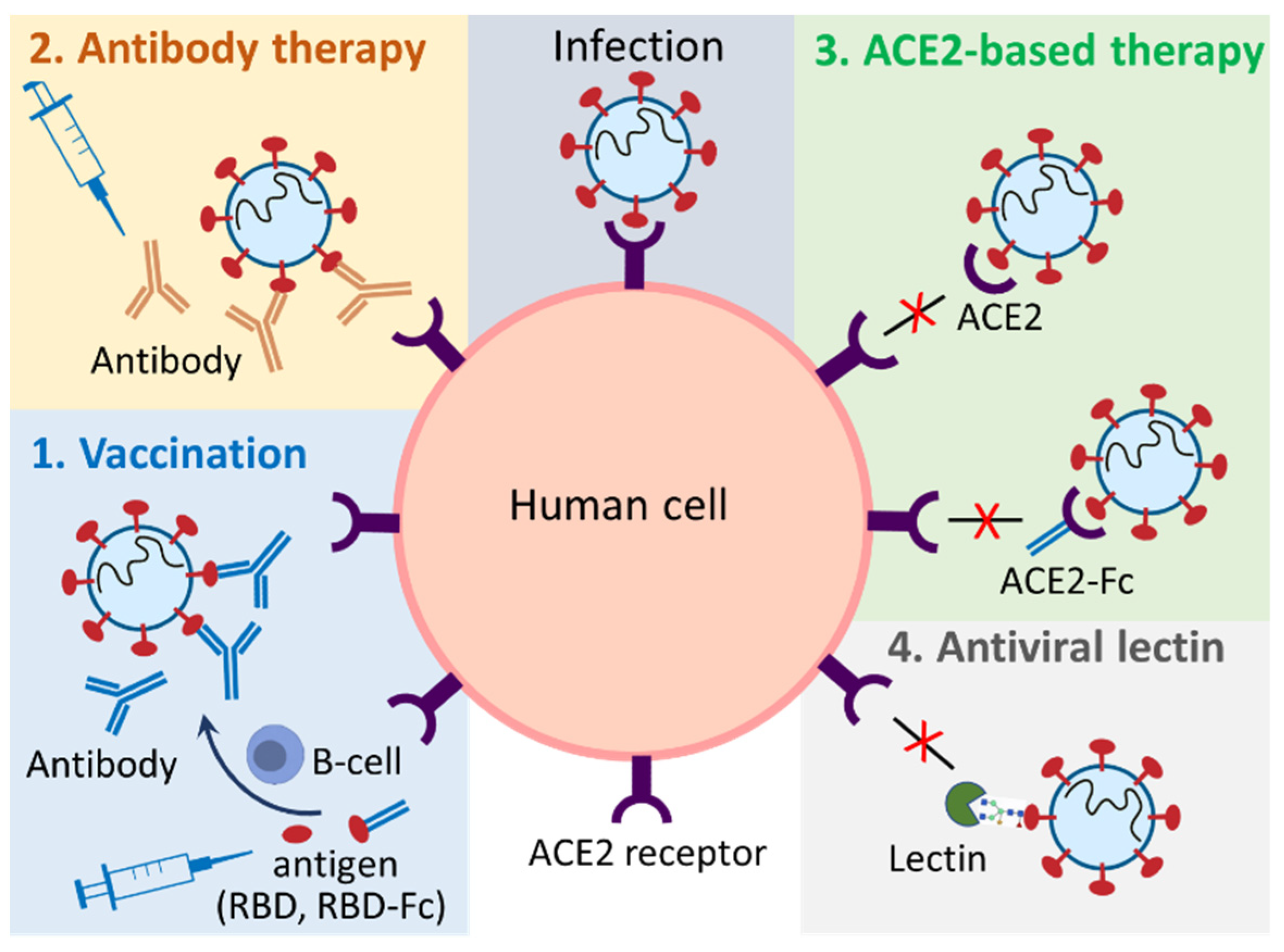
2. Plant-Produced Vaccines
| Table | Trade Name | Antigen | Plant | Manufacturer | Efficacy | Commercialization Progress | Source |
|---|---|---|---|---|---|---|---|
| Virus-like particles | Covifenz | S protein | N. benthamiana | Medicago | 69.5% to 78.8% (Phase III) |
Approved: Canada Phase III Trials: Argentina, Brazil, United Kingdom, USA |
[9][24][25] |
| KBP-201 | RBD | N. benthamiana | Kentucky Bioprocessing | 100% (K18-hACE2 mice) | Phase I/II Trials: USA | [25][26][27][28] | |
| IBIO-200, IBIO-201, and IBIO-202 | S protein | N. benthamiana | iBio, Inc. | n.d. | Pre-clinical trials | [21][29][30] | |
| n/a | S protein | N. benthamiana | n/a | n.d. | no | [31] | |
| Subunit | Baiya SARS-CoV-2 Vax 1 | RBD-Fc | N. benthamiana | Baiya Phytopharm | 100% (K18-hACE2 mice) |
Phase I Trials: Thailand | [25][32] |
| Baiya SARS-CoV-2 Vax 2 | RBD-Fc | N. benthamiana | Baiya Phytopharm | Unknown | Phase I Trials: Thailand | [25][32] | |
| n/a | RBD-Fc | N. benthamiana | n/a | n.d. | no | [10] | |
| n/a | RBD | N. benthamiana | n/a | n.d. | no | [33][34][35] | |
| n/a | S protein, RBD | Tobacco BY-2 and Medicago truncatula A17 cell | n/a | n.d. | no | [36] |
2.1. Plant-Produced Subunit Vaccines
2.2. Plant-Produced Virus-Like Particles Vaccines
3. Plant-Produced Antibodies
| Antibody Name | Plant | Affected Lineages | Neutralizing Capability (Neutralizing Titer *, IC50 † or NT100 ‡) | Source |
|---|---|---|---|---|
| CR3022 | N. benthamiana | Original strain | Fail to neutralize * | [34] |
| B38 | N. benthamiana | Unidentified | 640 at 0.492 µg/mL * | [54] |
| H4 | N. benthamiana | Unidentified | 40 at 5.45 µg/mL * | [54] |
| H4-IgG1-4 | N. benthamiana | Unidentified | 591 nM for H4-IgG3 ‡ | [55] |
| CA1 | N. benthamiana | Original strain, Delta |
9.29 nM: Original † 89.87 nM: Delta † |
[51] |
| CB6 | N. benthamiana | Original strain, Delta |
0.93 nM: Original † 0.75 nM: Delta † |
[51] |
| 11D7 | N. benthamiana | Original strain, Delta, Omicron |
25.37 µg/mL: Original † 59.52 µg/mL: Delta † 948.7 µg/mL: Omicron † |
[4] |
2.3. Plant-Produced Angiotensin-Converting Enzyme 2-Based Biologics
2.3.1. Plant-Produced Angiotensin-Converting Enzyme 2-Immunoadhesins
2.3.2. Plant-Produced Angiotensin-Converting Enzyme 2 and Angiotensin-Converting Enzyme 2-Based Chewing Gum
2.4. Plant Produced Antiviral Lectins
2.5. Challenges in Commercialization of Plant-Produced Biologics against SARS-CoV-2
This entry is adapted from the peer-reviewed paper 10.3390/life13030617
References
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359.
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol Adv. 2020, 40, 107519.
- Xu, J.; Towler, M.; Weathers, P.J. Platforms for plant-based protein production. In Bioprocessing of Plant In Vitro Systems, 1st ed.; Pavlov, A., Bley, T., Eds.; Springer International Publishing AG: Midtown Manhattan, NY, USA, 2016; pp. 1–40.
- Jugler, C.; Sun, H.; Nguyen, K.; Palt, R.; Felder, M.; Steinkellner, H.; Chen, Q. A novel plant-made monoclonal antibody enhances the synergetic potency of an antibody cocktail against the SARS-CoV-2 Omicron variant. Plant Biotechnol. J. 2022; published online ahead of print.
- Ruocco, V.; Strasser, R. Transient expression of glycosylated SARS-CoV-2 antigens in Nicotiana benthamiana. Plants 2022, 11, 1093.
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 2019, 10, 720.
- Tekoah, Y.; Shulman, A.; Kizhner, T.; Ruderfer, I.; Fux, L.; Nataf, Y.; Bartfeld, D.; Ariel, T.; Gingis-Velitski, S.; Hanania, U.; et al. Large-scale production of pharmaceutical proteins in plant cell culture-the protalix experience. Plant Biotechnol. J. 2015, 13, 1199–1208.
- Ward, B.J.; Makarkov, A.; Seguin, A.; Pillet, S.; Trepanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (>/=65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503.
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and safety of a recombinant plant-based adjuvanted COVID-19 vaccine. N. Engl. J. Med. 2022, 386, 2084–2096.
- Siriwattananon, K.; Manopwisedjaroen, S.; Shanmugaraj, B.; Rattanapisit, K.; Phumiamorn, S.; Sapsutthipas, S.; Trisiriwanich, S.; Prompetchara, E.; Ketloy, C.; Buranapraditkun, S.; et al. Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front. Plant Sci. 2021, 12, 682953.
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278.
- Mamedov, T.; Gurbuzaslan, I.; Yuksel, D.; Ilgin, M.; Mammadova, G.; Ozkul, A.; Hasanova, G. Soluble human angiotensin- converting enzyme 2 as a potential therapeutic tool for COVID-19 is produced at high levels in Nicotiana benthamiana plant with potent anti-SARS-CoV-2 activity. Front. Plant Sci. 2021, 12, 742875.
- Alfaleh, M.A.; Zawawi, A.; Al-Amri, S.S.; Hashem, A.M. David versus goliath: ACE2-Fc receptor traps as potential SARS-CoV-2 inhibitors. MAbs 2022, 14, 2057832.
- Cai, Y.; Xu, W.; Gu, C.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.; Jiang, S. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol. Sin. 2020, 35, 857–860.
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A comprehensive review of the protein subunit vaccines against COVID-19. Front. Microbiol. 2022, 13, 927306.
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2021, 12, 790121.
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636.
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376.
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220.
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643.
- Ortega-Berlanga, B.; Pniewski, T. Plant-based vaccines in combat against coronavirus diseases. Vaccines 2022, 10, 138.
- Peyret, H.; Steele, J.F.C.; Jung, J.W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing vaccines against enveloped viruses in plants: Making the impossible, difficult. Vaccines 2021, 9, 780.
- El Jaddaoui, I.; Al Idrissi, N.; Hamdi, S.; Wakrim, L.; Nejjari, C.; Amzazi, S.; Elouahabi, A.; Bakri, Y.; Ghazal, H. Plant-based vaccines against COVID-19 for massive vaccination in Africa. Front. Drug Deliv. 2022, 2, 909958.
- Medicago Covifenz COVID-19 Vaccine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html#a4 (accessed on 1 December 2022).
- COVID-19 Vaccine Development and Approvals Tracker. Available online: https://covid19.trackvaccines.org (accessed on 2 December 2022).
- KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04473690 (accessed on 1 December 2022).
- Demarco, J.K.; Royal, J.M.; Severson, W.E.; Gabbard, J.D.; Hume, S.; Morton, J.; Swope, K.; Simpson, C.A.; Shepherd, J.W.; Bratcher, B.; et al. CoV-RBD121-NP vaccine candidate protects against symptomatic disease following SARS-CoV-2 challenge in K18-hACE2 mice and induces protective responses that prevent COVID-19-associated immunopathology. Vaccines 2021, 9, 1346.
- Royal, J.M.; Simpson, C.A.; McCormick, A.A.; Phillips, A.; Hume, S.; Morton, J.; Shepherd, J.; Oh, Y.; Swope, K.; De Beauchamp, J.L.; et al. Development of a SARS-CoV-2 vaccine candidate using plant-based manufacturing and a tobacco mosaic virus-like nano-particle. Vaccines 2021, 9, 1347.
- Maharjan, P.M.; Choe, S. Plant-based COVID-19 vaccines: Current status, design, and development strategies of candidate vaccines. Vaccines 2021, 9, 992.
- Uthaya Kumar, A.; Kadiresen, K.; Gan, W.C.; Ling, A.P.K. Current updates and research on plant-based vaccines for coronavirus disease 2019. Clin. Exp. Vaccine Res. 2021, 10, 13.
- Balieu, J.; Jung, J.W.; Chan, P.; Lomonossoff, G.P.; Lerouge, P.; Bardor, M. Investigation of the N-glycosylation of the SARS-CoV-2 S protein contained in VLPs produced in Nicotiana benthamiana. Molecules 2022, 27, 5119.
- Shanmugaraj, B.; Khorattanakulchai, N.; Panapitakkul, C.; Malla, A.; Im-Erbsin, R.; Inthawong, M.; Sunyakumthorn, P.; Hunsawong, T.; Klungthong, C.; Reed, M.C.; et al. Preclinical evaluation of a plant-derived SARS-CoV-2 subunit vaccine: Protective efficacy, immunogenicity, safety, and toxicity. Vaccine 2022, 40, 4440–4452.
- Mamedov, T.; Yuksel, D.; Ilgin, M.; Gurbuzaslan, I.; Gulec, B.; Yetiskin, H.; Uygut, M.A.; Islam Pavel, S.T.; Ozdarendeli, A.; Mammadova, G.; et al. Plant-produced glycosylated and in vivo deglycosylated receptor binding domain proteins of SARS-CoV-2 Induce potent neutralizing responses in mice. Viruses 2021, 13, 1595.
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Smith, D.R.; et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 17698.
- Demone, J.; Maltseva, M.; Nourimand, M.; Nasr-Sharif, M.; Galipeau, Y.; Alarcon, E.I.; Langlois, M.A.; MacLean, A.M. Scalable agroinfiltration-based production of SARS-CoV-2 antigens for use in diagnostic assays and subunit vaccines. PLoS ONE 2022, 17, e0277668.
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 spike protein and its receptor binding domain in plant cell suspension cultures. Front. Plant Sci. 2022, 13, 995429.
- Larkin, H.D. Novavax COVID-19 vaccine booster authorized. JAMA 2022, 328, 2101.
- Granwehr, B.P. In adults who had not had COVID-19, Novavax vaccine had 90% efficacy at >/=7 d after the second dose. Ann. Intern. Med. 2022, 175, JC52.
- Marabotti, C. Efficacy and effectiveness of COVID-19 vaccine—Absolute vs. relative risk reduction. Expert Rev. Vaccines 2022, 21, 873–875.
- Mardanova, E.S.; Kotlyarov, R.Y.; Ravin, N.V. High-yield production of receptor binding domain of SARS-CoV-2 linked to bacterial flagellin in plants using self-replicating viral vector pEff. Plants 2021, 10, 2682.
- Khorattanakulchai, N.; Srisutthisamphan, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Rattanapisit, K.; Panapitakkul, C.; Kemthong, T.; Suttisan, N.; Malaivijitnond, S.; Thitithanyanont, A.; et al. A recombinant subunit vaccine candidate produced in plants elicits neutralizing antibodies against SARS-CoV-2 variants in macaques. Front. Plant Sci. 2022, 13, 901978.
- Khorattanakulchai, N.; Manopwisedjaroen, S.; Rattanapisit, K.; Panapitakkul, C.; Kemthong, T.; Suttisan, N.; Srisutthisamphan, K.; Malaivijitnond, S.; Thitithanyanont, A.; Jongkaewwattana, A.; et al. Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern. J. Med. Virol. 2022, 94, 4265–4276.
- Phoolcharoen, W.; (Chulalongkorn University, Bangkok, Thailand). Personal communication, 2022.
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59.
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83.
- Schwarz, B.; Uchida, M.; Douglas, T. Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology. Adv. Virus Res. 2017, 97, 1–60.
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078.
- iBio Reports Preliminary Unaudited Fiscal Year 2022 Financial Results and Provides Corporate Update. Available online: https://www.globenewswire.com/news-release/2022/09/27/2523812/0/en/iBio-Reports-Preliminary-Unaudited-Fiscal-Year-2022-Financial-Results-and-Provides-Corporate-Update.html#:~:text=Preliminary%20Unaudited%20Financial%20Results%3A,comparable%20period%20in%20fiscal%202021 (accessed on 1 December 2022).
- Pipeline Therapeutic Candidates. Available online: https://ibioinc.com/pipeline/ (accessed on 2 December 2022).
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30.
- Jugler, C.; Sun, H.; Grill, F.; Kibler, K.; Esqueda, A.; Lai, H.; Li, Y.; Lake, D.; Chen, Q. Potential for a plant-made SARS-CoV-2 neutralizing monoclonal antibody as a synergetic cocktail component. Vaccines 2022, 10, 772.
- Chen, Q. Development of plant-made monoclonal antibodies against viral infections. Curr. Opin. Virol. 2022, 52, 148–160.
- Ma, J.K.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015, 13, 1106–1120.
- Shanmugaraj, B.; Rattanapisit, K.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Monoclonal antibodies B38 and H4 produced in Nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front. Plant Sci. 2020, 11, 589995.
- Kallolimath, S.; Sun, L.; Palt, R.; Stiasny, K.; Mayrhofer, P.; Gruber, C.; Kogelmann, B.; Chen, Q.; Steinkellner, H. Highly active engineered IgG3 antibodies against SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2107249118.
- Jung, J.W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-based expression and characterization of SARS-CoV-2 virus-like particles presenting a native spike protein. Plant Biotechnol. J. 2022, 20, 1363–1372.
- Wang, Z.; Yang, L.; Song, X.Q. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355.
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E., Jr.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495.
- Cohen-Dvashi, H.; Weinstein, J.; Katz, M.; Eilon-Ashkenazy, M.; Mor, Y.; Shimon, A.; Achdout, H.; Tamir, H.; Israely, T.; Strobelt, R.; et al. Anti-SARS-CoV-2 immunoadhesin remains effective against Omicron and other emerging variants of concern. iScience 2022, 25, 105193.
- Chamow, S.M.; Ashkenazi, A. Immunoadhesins: Principles and applications. Trends Biotechnol. 1996, 14, 52–60.
- Chen, Y.; Sun, L.; Ullah, I.; Beaudoin-Bussieres, G.; Anand, S.P.; Hederman, A.P.; Tolbert, W.D.; Sherburn, R.; Nguyen, D.N.; Marchitto, L.; et al. Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities. Sci. Adv. 2022, 8, eabn4188.
- Yasui, F.; Kohara, M.; Kitabatake, M.; Nishiwaki, T.; Fujii, H.; Tateno, C.; Yoneda, M.; Morita, K.; Matsushima, K.; Koyasu, S.; et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology 2014, 454–455, 157–168.
- Siriwattananon, K.; Manopwisedjaroen, S.; Kanjanasirirat, P.; Budi Purwono, P.; Rattanapisit, K.; Shanmugaraj, B.; Smith, D.R.; Borwornpinyo, S.; Thitithanyanont, A.; Phoolcharoen, W. Development of plant-produced recombinant ACE2-Fc fusion protein as a potential therapeutic agent against SARS-CoV-2. Front. Plant Sci. 2021, 11, 604663.
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070.
- Glasgow, A.; Glasgow, J.; Limonta, D.; Solomon, P.; Lui, I.; Zhang, Y.; Nix, M.A.; Rettko, N.J.; Zha, S.; Yamin, R.; et al. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 28046–28055.
- Higuchi, Y.; Suzuki, T.; Arimori, T.; Ikemura, N.; Mihara, E.; Kirita, Y.; Ohgitani, E.; Mazda, O.; Motooka, D.; Nakamura, S.; et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat. Commun. 2021, 12, 3802.
- Bernardi, A.; Huang, Y.; Harris, B.; Xiong, Y.; Nandi, S.; McDonald, K.A.; Faller, R. Development and simulation of fully glycosylated molecular models of ACE2-Fc fusion proteins and their interaction with the SARS-CoV-2 spike protein binding domain. PLoS ONE 2020, 15, e0237295.
- Mou, H.; Quinlan, B.D.; Peng, H.; Liu, G.; Guo, Y.; Peng, S.; Zhang, L.; Davis-Gardner, M.E.; Gardner, M.R.; Crynen, G.; et al. Mutations derived from horseshoe bat ACE2 orthologs enhance ACE2-Fc neutralization of SARS-CoV-2. PLoS Pathog. 2021, 17, e1009501.
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265.
- Tada, T.; Fan, C.; Chen, J.S.; Kaur, R.; Stapleford, K.A.; Gristick, H.; Dcosta, B.M.; Wilen, C.B.; Nimigean, C.M.; Landau, N.R. An ACE2 microbody containing a single immunoglobulin Fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep. 2020, 33, 108528.
- Tsai, T.I.; Khalili, J.S.; Gilchrist, M.; Waight, A.B.; Cohen, D.; Zhuo, S.; Zhang, Y.; Ding, M.; Zhu, H.; Mak, A.N.; et al. ACE2-Fc fusion protein overcomes viral escape by potently neutralizing SARS-CoV-2 variants of concern. Antivir. Res. 2022, 199, 105271.
- Castilho, A.; Schwestka, J.; Kienzl, N.F.; Vavra, U.; Grunwald-Gruber, C.; Izadi, S.; Hiremath, C.; Niederhofer, J.; Laurent, E.; Monteil, V.; et al. Generation of enzymatically competent SARS-CoV-2 decoy receptor ACE2-Fc in glycoengineered Nicotiana benthamiana. Biotechnol. J. 2021, 16, e2000566.
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol. Ther. 2022, 30, 1966–1978.
- Daniell, H.; Nair, S.K.; Guan, H.; Guo, Y.; Kulchar, R.J.; Torres, M.D.; Shahed-Al-Mahmud, M.; Wakade, G.; Liu, Y.M.; Marques, A.D. Debulking different Corona (SARS-CoV-2 delta, omicron, OC43) and influenza (H1N1, H3N2) virus strains by plant viral trap proteins in chewing gums to decrease infection and transmission. Biomaterials 2022, 288, 121671.
- Ganesan, P.K.; Kulchar, R.J.; Kaznica, P.; Montoya-Lopez, R.; Green, B.J.; Streatfield, S.J.; Daniell, H. Optimization of biomass and target protein yield for Phase III clinical trial to evaluate Angiotensin Converting Enzyme 2 expressed in lettuce chloroplasts to reduce SARS-CoV-2 infection and transmission. Plant Biotechnol. J. 2022; published online ahead of print.
- Ghafoor, D.; Ahmed, S.; Zaman, N. Lectins; a hope of treatment for COVID-19. Am. J. Biomed. Sci. Res. 2021, 12, 280–282.
- Ahmed, M.N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 2022, 146, 112507.
- Naik, S.; Kumar, S. Lectins from plants and algae act as anti-viral against HIV, influenza and coronaviruses. Mol. Biol. Rep. 2022, 49, 12239–12246.
- Barre, A.; Van Damme, E.J.M.; Simplicien, M.; Le Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rouge, P. Man-Specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical perspectives. Cells 2021, 10, 1619.
- Nascimento da Silva, L.C.; Mendonca, J.S.P.; de Oliveira, W.F.; Batista, K.L.R.; Zagmignan, A.; Viana, I.F.T.; Dos Santos Correia, M.T. Exploring lectin-glycan interactions to combat COVID-19: Lessons acquired from other enveloped viruses. Glycobiology 2021, 31, 358–371.
- Chan, J.F.; Oh, Y.J.; Yuan, S.; Chu, H.; Yeung, M.L.; Canena, D.; Chan, C.C.; Poon, V.K.; Chan, C.C.; Zhang, A.J.; et al. A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Cell Rep. Med. 2022, 3, 100774.
- Liu, Y.M.; Shahed-Al-Mahmud, M.; Chen, X.; Chen, T.H.; Liao, K.S.; Lo, J.M.; Wu, Y.M.; Ho, M.C.; Wu, C.Y.; Wong, C.H.; et al. A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016.
- Lee, C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: From discovery to clinical application. Mar. Drugs 2019, 17, 567.
- Ahan, R.E.; Hanifehnezhad, A.; Kehribar, E.S.; Oguzoglu, T.C.; Foldes, K.; Ozcelik, C.E.; Filazi, N.; Oztop, S.; Palaz, F.; Onder, S.; et al. A highly potent SARS-CoV-2 blocking lectin protein. ACS Infect. Dis. 2022, 8, 1253–1264.
- Lokhande, K.B.; Apte, G.R.; Shrivastava, A.; Singh, A.; Pal, J.K.; Swamy, K.V.; Gupta, R.K. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 3880–3898.
- Nabi-Afjadi, M.; Heydari, M.; Zalpoor, H.; Arman, I.; Sadoughi, A.; Sahami, P.; Aghazadeh, S. Lectins and lectibodies: Potential promising antiviral agents. Cell Mol. Biol. Lett. 2022, 27, 37.
- Gecchele, E.; Merlin, M.; Brozzetti, A.; Falorni, A.; Pezzotti, M.; Avesani, L. A comparative analysis of recombinant protein expression in different biofactories: Bacteria, insect cells and plant systems. J. Vis. Exp. 2015, 97, 52459.
- Gomord, V.; Fitchette, A.C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 2010, 8, 564–587.
