There is a significant potential to increase the sustainability of the fishing and aquaculture industries through the maximization of the processing of byproducts. Enzymatic hydrolysis provides an opportunity to valorize downstream fish industry byproducts for the production of protein hydrolysates (FPH) as a source of bioactive peptides (BAP) with health benefits. Deteriorative oxidative reactions may occur during the enzymatic hydrolysis of byproducts, influencing the safety or bioactivities of the end product. Lipid oxidation, autolysis mediated by endogenous enzymes in viscera, protein degradation, and formation of low-molecular-weight metabolites are the main reactions that are expected to occur during hydrolysis and need to be controlled.
1. Introduction
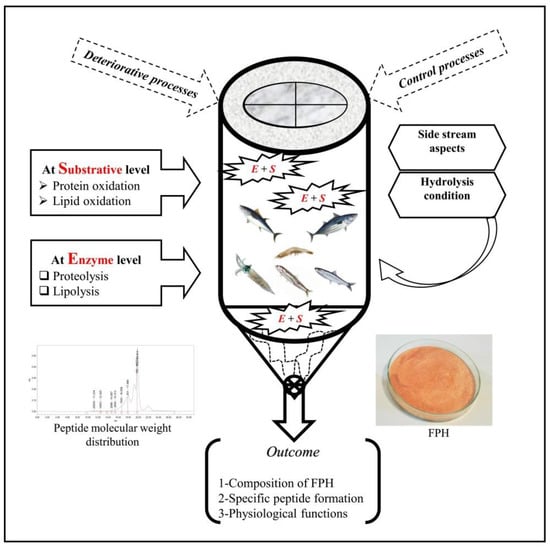
During enzymatic hydrolysis, heating the solution to achieve the optimal activity for proteases, changes of pH, the presence of pro-oxidants (i.e., hemoglobin and metal ions) and unsaturated lipids in addition to initial quality of byproducts can cause oxidation, which should be studied at both substrative and hydrolysis levels (Figure 1).
Figure 1. Main oxidative processes, control measures, and properties of FPH from byproducts of the seafood industry.
2. Lipid Oxidation Products
Oxygen is vital for oxidative reactions. The solubility of oxygen is higher in oils than in water [
54]. Lipids in byproducts gradually release into the hydrolysis solution, causing oxidation. In Sind sardine, hydrolysis of pretreated fish by 5% Alcalase in the presence of nitrogen gas (N
2) significantly decreased lipid oxidation along with having higher DH and antioxidant activity for the FPH [
22]. Decomposition of formed peroxides during enzymatic hydrolysis [
36,
48] by pro-oxidative metal ions is a driving factor for lipid oxidation. Metal-catalyzed hydroperoxides degradation generally takes place via cleavage of an oxygen–oxygen bond in the peroxide (LOOH), producing some highly reactive alkoxyl lipid radicals (LO
•) and hydroxyl ions (OH
−). LO
• further degrades rapidly by carbon-carbon cleavage on either side of the radical to form volatile decomposition products or off-odors [
54]. In addition, the initial oxidative status of lipids in byproducts is very important for the extent of lipid oxidation during hydrolysis as well as the sensory quality and oxidative stability of FPH. The TBARS, PV, and undesirable fishy odor in FPH produced from fresh Nile tilapia were significantly lower than that produced from non-fresh fish [
55].
3. Blood Components and Degradation
Fish byproducts contain considerable amounts of blood containing hemoglobin (Hb) and iron as pro-oxidants. These components can react with each other during enzymatic reactions [
56]. The brown color development during the enzymatic hydrolysis of byproducts may be explained by the auto-oxidation of Hb and changes of Fe
2+ to Fe
3+ to form metHb, which has increased pro-oxidant activity compared to its reduced form. Dissociation of the iron-protoporphyin IX moiety (or heme) from metHb is relevant in the context of lipid oxidation. Iron-protoporphyin IX released from metHb intercalates within the phospholipid bilayer of cellular membranes facilitating the breakdown of any lipid hydroperoxides (LOOH) formed to alkoxyl and peroxyl radicals that propagate lipid oxidation [
56]. Hemoglobin (5 and 20 μM/kg) and iron (100 or 200 μM) in the presence of fish oil (5%,
v/
v) induced oxidation during hydrolysis of cod using a commercial protease at 3%
w/
v and 36 °C. In frames, trimmings, and heads with remaining fish meat, Hb might contribute to both lipid and protein oxidation during hydrolysis, the same as can happen with fillets, which could have impacts on the structural properties and oxidation of peptides [
21]. Larsson and Undeland [
57] investigated Hb-mediated lipid and protein oxidation in washed cod mince and found an increased peroxide value (PV), rancid odor, protein carbonylation, and insolubilisation in the presence of 20 μmol/L Hb. The higher Hb and iron in herring heads compared to other fractions of byproducts, including frames, belly flap, and viscera, led to greater lipid oxidation during ice storage [
25]. DeoxyHb could easily react with O
2 to rapidly form metHb and superoxide radicals that can dismutate to hydrogen peroxide (H
2O
2) and facilitate lipid oxidation [
56]. Therefore, reducing oxygen in reactors may decrease undesirable oxidative reactions during enzymatic hydrolysis. With tuna byproducts, hydrolysis in the presence of nitrogen gas decreased TBA, off-odor, brown discoloration, and bitterness in the FPH [
58].
4. Protein Carbonyls, Amino Acid Degradation, and Solubility
The gastrointestinal tract and internal organs could be exposed to the cytotoxic and mutagenic potential of harmful free radicals formed in oxidized proteins and peptides such as alkyl, peroxyl, and alkoxyl radicals and carbonyls [
59]. Oxidation products may interact with other components, influencing FPH quality, and this can influence the oxidative deteriorations during enzymatic hydrolysis where all components of byproducts are present at their original concentrations, unless subjected to substrate pretreatment [
7]. Rainbow trout byproducts subjected to washing using distilled water (BPW-FPI) or distilled water prior to calcium chloride and citric acid washing (W-CaCi-BPFPI) had a lower content of protein carbonyls in the dried FPH [
45]. Secondary protein carbonyls can be introduced into proteins using a covalent linkage of lipid carbonyls (i.e., protein-bound malondialdehyde (MDA)) [
48]. Both lipid and protein oxidation can occur during the enzymatic hydrolysis of byproducts that makes the process more biochemically complicated. Oxidized peptides may lose their nutritional value due to the destruction of essential amino acids and impaired digestibility [
60].
The solubility of peptides influences their functional properties (including emulsifying and foaming properties, and water holding capacity) for food applications [
53]. During enzymatic hydrolysis, after unfolding the protein structure, polar (but generally uncharged) and non-polar amino acid groups inside the protein come to the protein surface, and then polar residues interact with water molecules to form hydrogen bonds and electrostatic interactions, thereby affecting solubility [
7,
45]. The reduced solubility (62.6%) of FPH produced from oxidized rainbow trout heads (FPH-OX) was associated with higher levels of protein carbonyls (5.14 nmol/mg protein) showing that protein oxidation and aggregation were occurring during enzymatic hydrolysis [
52].
5. Autolysis Mediated by Endogenous Enzymes
Visceral and muscle proteases are the main groups of endogenous enzymes in byproducts, contributing to the deterioration (i.e., autolysis) of byproducts with inappropriate storage, which then may increase oxidative deterioration during enzymatic hydrolysis. In frames, heads, and trimmings, muscle proteases, including lysosomal cathepsins, alkaline proteases, and neutral proteases, are present and show high capacity for hydrolyzing myofibrillar proteins at neutral or slightly alkaline pH, especially with improper handling and storage (i.e., high storage temperatures and longer times). In viscera, four main groups of proteases are available in the stomach, pyloric caeca, and intestine. These include acidic/aspartyl proteases (pepsins), serine proteases (such as trypsin and chymotrypsin), cysteine (thiol) proteases, and metalloprotease. These enzymes could be activated during enzymatic hydrolysis with optimal conditions for their activity (e.g., pH and temperature) accelerating the reactions unless deactivated by heating to avoid autolytic hydrolysis [
3]. Endogenous enzymes along with bacterial enzymes are responsible for the process of autolysis in byproducts after a fish’s death. Consequently, the degradation of proteins and the development of off odors and off flavors takes place that lowers the quality of byproducts for enzymatic hydrolysis. Action of lipases leads to the formation of degradation products such as free fatty acids that are responsible for oxidation during hydrolysis and oxidative instability of FPH [
61].
This entry is adapted from the peer-reviewed paper 10.3390/pr11020543