Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pathology
Sepsis is a life-threatening condition characterized by an uncontrolled inflammatory response to an infectious agent and its antigens. Immune cell activation against the antigens causes severe distress that mediates a strong inflammatory response in vital organs. Sepsis is responsible for a high rate of morbidity and mortality in immunosuppressed patients.
- monoclonal antibody therapy
- sepsis
- septic shock
1. Introduction
The definition of sepsis has been a concern of constant evolution and fine-tuning [1]. With advancing knowledge on the pathogenic mechanism of sepsis, currently “sepsis is well-defined as a serious, potentially life threatening, organic dysfunction initiated by an inadequate or dysregulated host response to infection” [2,3]. According to a global analysis, approximately 48.9 million people are estimated to be affected by sepsis yearly and 11 million deaths are estimated to occur by sepsis [4]. Despite the advances in understanding the pathology of sepsis and the development of its treatments, sepsis remains the leading cause of mortality worldwide [5]. Sepsis is a multifaceted disorder involving inflammation and anti-inflammation disbalance leading to the unregulated widespread release of inflammatory mediators, cytokines, and pathogen-related molecules leading to systemwide organ dysfunction [6]. Although the knowledge of the etiology, pathophysiology, and immunology has improved drastically over the past few years, the knowledge regarding the successful management of the same remains limited [1]. Early diagnosis followed by immediate treatment entails the success rate of treatment in sepsis [7]. Currently, treatment for sepsis and septic shock deeply relies on fluid resuscitation and other general supportive measures along with broad-spectrum antibiotics administration [8].
With the advent of antibiotics in the 20th century, they remain [9] the mainstay for the treatment of bacterial infection [10]. However, empirical use of broad-spectrum antibiotics has been associated with increased mortality and the development of antibiotic resistance [11]. The selective pressure of the unsupervised use of antibiotics has already driven bacteria to develop resistant genes against antibiotics [12]. The rise of antibiotic resistance is notably one of the biggest threats of the 21st century leading to therapeutic failure in the field of infectious diseases [13]. In fact, the dawn of extensively pandrug-resistant (PDR), multidrug-resistant (MDR), and extensively drug-resistant (XDR) strains of ESKAPE pathogens (Acinetobacter baumannii, Enterobacter spp., Enterococcus faecium, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus) combined with the rising gap between the development of antibiotic resistance and novel antibiotics has compelled researchers to shift focus on devising alternative innovations for combating bacterial infection [14]. Of such strategies, monoclonal antibiotics (mAbs) stand out as a promising avenue. Serum treatment was one of the effective ways used to treat bacterial infections early in the 1890s. However, limited spectrum and safety concerns such as allergy and cross-reaction led to its discontinuation [15]. The development of hybridoma technology in 1975 revolutionized the field of research and medicine, garnering the 1984 Nobel prize recognition in physiology and medicine [16,17]. With the introduction of hybridoma technology, mAbs have had a profound impact on immunotherapy, providing large-scale production of pure antibodies with improved specificity and reduced immunogenicity [16].
The major obstacle hindering the successful translation of drug candidates in sepsis is the complexity of disease progression that leads to a heterogeneous population with variable underlying clinical presentation, comorbidities, and prognosis abilities [18]. Therefore, monoclonal antibodies targeted against a particular sepsis biomarker present a viable therapeutic option [18]. Monoclonal antibodies may be beneficial in the treatment of sepsis by either directly impeding the growth of the pathogen or by immunomodulation. Initially overshadowed by viral infection and cancer, the application of mAbs in the treatment of bacterial infection is fairly a new approach with only a few FDA-approved drugs [15]. However, it has garnered considerable attention and several promising candidates for mAbs are undergoing clinical trials at present. The present manuscript aims to provide a comprehensive journey of establishing mAbs as a potential alternative therapy for sepsis by highlighting milestone achievements that were ratified by the Federal and Drug Administration (FDA) and those that failed clinical trials but have significantly contributed to the knowledge base.
2. Pathophysiology of Sepsis
Sepsis is a multifaceted chaos of wavering balance between inflammation and anti-inflammation leading to the unregulated widespread release of inflammatory mediators, cytokines, and pathogen-related molecules [6]. This dysregulated host response further activates coagulation and complements cascades that often result in death accompanied by multiple organ dysfunction [19].
The initial activation of the host immune system is mediated by the binding of invading pathogens by pattern-recognition receptors (PRR) on the surface of antigen-presenting cells (APCs) [20]. The PRR-like toll-like receptors (TLR), C-type lectin receptors (CLR’s), retenoic-inducible gene I (RIG-I), and nucleotide-binding oligomerization domain (Nod)-like receptors recognize pathogen-associated molecular patterns (PAMPs) [21,22] and/or host-derived damaged associated molecular patterns (DAMPs) [23]. Murine and mouse models of sepsis revealed that TLR 2, 3, 4, 7 and 9 are involved in the pathogenesis of sepsis by mediating the host’s innate immune response [24,25,26]. Upon recognition of PAMP and damps by TLRs, TLRs initiate transcription of genes involved in inflammation and adaptive immunity through activation of transcription factors such as nuclear factor-κB (NF-κB), activator protein (AP)-1, and mitogen-activated protein kinase (MAPK). This activation of “early genes” leads to the production of pro-inflammatory cytokines such as interleukins IL-8, 12,18, interferons (INFs), and tumor necrosis factor alpha (TNF-α). The pro-inflammatory cytokines thus produced, downstream recruit a torrent of other inflammatory cytokines and chemokines. Components of adaptive immunity are suppressed during the process [27] (Figure 1).
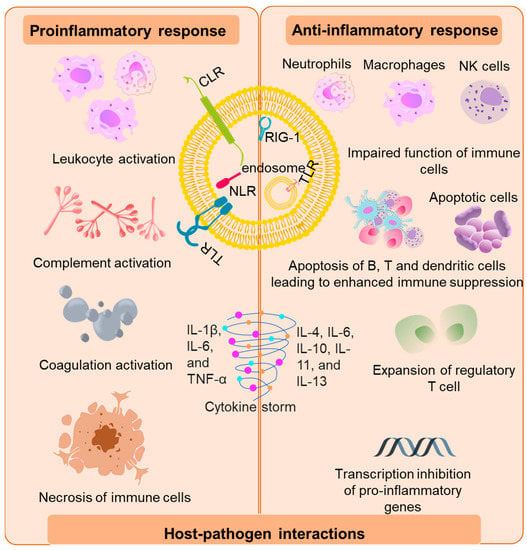
Figure 1. Immunopathogenesis of sepsis. The initial host response is triggered by the binding of bacterial virulence factors to the various pattern recognition receptors such as toll-like receptors (TLR), C-type lectin receptors (CLRs), nucleotide-binding oligomerization domain (Nod)-like receptors and retenoic-inducible gene I (RIG-I). The pro-inflammatory response is marked by the activation of leukocytes which further activates components of the complement and coagulation systems and vascular endothelium via secretion of mediators such as cytokines, reactive oxygen species, and proteases. This led to release of damaged associated molecular patterns (DAMPs) that further exacerbate the pro-inflammatory response. The anti-inflammatory response is represented by impaired function of immune cells, abnormal level of apoptosis, exhaustion of B cells, T cells, and dendritic cells due to negative regulation of TLR signaling and inhibition of pro-inflammatory genes leading to “immunoparalysis”.
This overwhelming cytokine storm may initially be beneficial in the prognosis of sepsis; however, it ultimately results in progressive organ failure and finally death [6]. In fact, patients in the later stages of sepsis display “immunoparalysis”, a state of chronic immunosuppression [28]. Immunoparalysis is an adjudication of enhanced apoptosis, pyroptosis of immune cells merged with the exhaustion of T cells that marks a patient’s vulnerability to secondary nosocomial infections and reactivation of viruses [18,29,30]. The clinical decline of multiple organs and the development of intravascular thrombosis have been associated with the overproduction or inadequate degradation of immune cell-extracellular traps originally forged to trap and devour pathogens [30,31].
Although sepsis is recognized as an interplay of dysregulated host immune response to infection, clinical representation of the same remains highly individualized making clinical diagnostics a challenge [1]. This may be due to factors such as genetics and the knowledge gap in the understanding of sepsis pathophysiology [32].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11030765
This entry is offline, you can click here to edit this entry!
