Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Electrochemistry
MXenes have been considered to be potential building blocks for composites for use in energy storage applications due to their distinctive 2D wafer structure and superior electrical conductivity. MXenes have been combined with multiple active ingredients, including metal oxides and conductive polymers, to produce a synergistic effect. The synthesis method of MXene shows various surface termini and topographies with different energy storage properties, and there have been multiple studies examining surface modification, stoichiometric ratio, and electrode composition control.
- synthesis method
- 2D MXene
- electrolyte
- MXene-based electrode
1. Control of Size of MXene Flake
The morphology and structure of MXenes are strongly influenced by the corrosivity of the synthesis conditions. For example, prolonging the etching time produces a more open structure than the HF etching of Ti3AlC2, which produces an accordion-like Ti3C2Tx (MXene). Ti3C2 with a corrosion time of 216 h has a deformed octahedral structure that loses more Ti and C contact on the surface than Ti3C2 with a short corrosion time of 24 h, thus giving it significantly better capacitive achievement [81,82], as shown in Figure 12. However, despite the higher ionic conductivity and easier access to ion diffusion pathways, the smaller MXene fragments have higher electrical conductivity due to the higher resistance to intersurface contact. Kayali et al. [83] examined how the side diameters of MXenes affect their electrochemical properties. In addition, Mustafa et al. [71] demonstrated that rational design electrodes are fabricated by mixing MXene with an aqueous solution of chloroauric acid (HAuCl4). As shown in Figure 12g,h, the etching time had an influential effect on electrochemical properties of MXene. The symmetric supercapacitors made of MXene and AuNPs have shown exceptional specific capacitance of 696.67 F g−1 at 5 mV s−1 in 3 M H2SO4 electrolyte, and they can sustain 90% of their original capacitance for 5000 cycles (in Figure 13). The highest energy and power density of this device, which operates within a 1.2 V potential window, are 138.4 Wh kg−1 and 2076 W kg−1, respectively. From topography analysis, it is clearly shown that Au NPS are homogeneously located on a MXene nanosheet; therefore, the Au NPs served as the best intercalators in the hybrid structure [84]. The thickness of the MXene/AuNPs composite film had stacked layer with a 12 μm thickness.
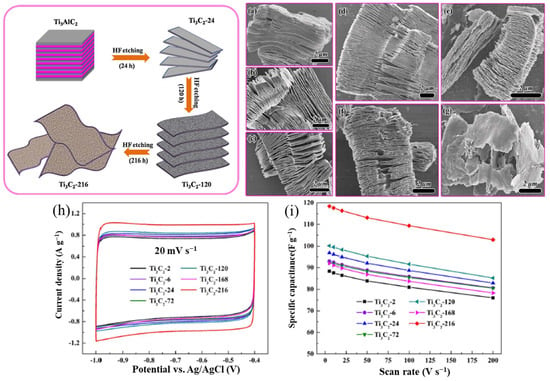
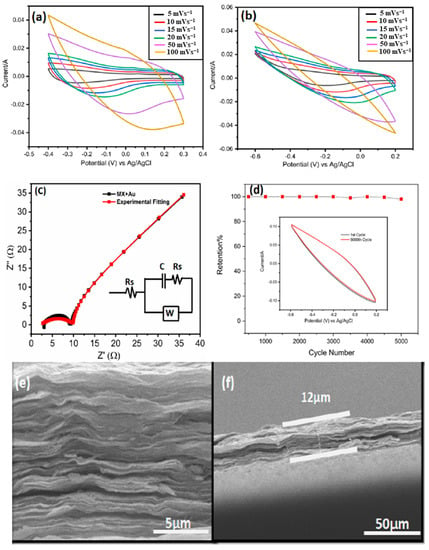
Figure 13. (a) CV curves of MXene at different scan rates in 3-electrode setup; (b) CV curves of MX/AuNPs in 1 M H2SO4 at different scan rates in 3-electrode setup; (c) Nyquist plots of MX/AuNPs with inset showing the equivalent circuit model for the Nyquist plots; (d) MX/AuNPs electrode showing excellent cyclic stability with 98% capacitance retention at 100 mV s−1 1M H2SO4 after 5000 cycles; (e) cross-sectional image of the MX/AuNPs composite film demonstrating stacked layered structure; (f) cross-sectional image showing the thickness of the composite film [84].
2. Category of Composition
Table 1 summarizes most of the previous studies examining Ti3C2Tx and other MXenes for energy storage applications or supercapacitor electrodes. Ti2CTx MXenes [85,86] deliver outstanding outcomes. Compared to carbides, nitride-based MXene exhibits higher electrical conductivity [87]. Accordion-structured, -O/-OH-terminated Ti2NTx nanolayers are obtained by synthesizing the Ti2AlN (MAX) phase by oxygen-assisted molten salt followed by HCl treatment.
Table 1. Capacitive capabilities of different MXene electrode materials for SCs.
| Versatility | Synthesis | Electrode Assembly |
Electrolyte | Capacitance | Cycle Stability |
Ref. |
|---|---|---|---|---|---|---|
| Ti3C2Tx | HF etching Ti3AlC2 and DMSO delamination | Filtrating into film | 1 M KOH | 350 F cm−3 | no degradation (10,000 cycles) |
[41] |
| Ti3C2Tx | HCl/HF etching Ti3AlC2 | Rolling | 1 M H2SO4 | 900 F cm−3 or 245 F g−1 | no degradation (10,000 cycles) |
[39] |
| Ti3C2Tx | Lewis acidic molten salt etching Ti3SiC2 | Rolling | 1 M LiPF6- EC/DMC |
738 C g−1 or 205 mAh g−1 | 90% (2400 cycles) |
[31] |
| Ti3CTx | HF etching Ti2AlC | Rolling | 1 M KOH | 517 F cm−3 | no degradation (3000 cycles) |
[85] |
| Ti3NTx | oxygen-assisted molten salt etching Ti2AlN2 and HCl treatment | Coating | 1 M MgSO4 | 201 F g−1 | 140% (1000 cycles) |
[88] |
| V2C | HF etching V2AlC and TMAOH delamination | Rolling | 1 M H2SO4 | 487 F g−1 | 83% (10,000 cycles) | [89] |
| V4C3Tx | HF etching V4AlC3 | Coating | 1 M H2SO4 | ~209 F g−1 | 97.23% (10,000 cycles) | [90] |
| Nb4C3Tx | HF etching Nb4AlC3 and TMAOH delamination |
Rolling | 1 M H2SO4 | 1075 F cm−3 | 76% (5000 cycles) | [91] |
| Ta4C3 | HF etching Ta4AlC3 | Coating | 0.1 M H2SO4 | 481 F g−1 | 89% (2000 cycles) | [92] |
| Mo2CTx | HF etching Mo2Ga2C and TBAOH delamination | Filtrating into film | 1 M H2SO4 | 700 F cm−3 | no degradation (10,000 cycles) |
[36] |
| Mo2TiC2Tx | HF etching and DMSO delamination Mo2TiAlC2 | Rolling | 1 M H2SO4 | 413 F cm−3 | no degradation (10,000 cycles) | [43] |
Nanolayered Ti2NTx MXene with -O/-OH surface terminations exhibited a capacitance of 200 F g−1 at a scan rate of 2 mV s−1 in 1 M MgSO4 electrolyte, and this MXene material was prepared by HCl treatment on Ti2AlN-MAX phase. In addition to traditional Ti-based MXene materials, members of the MXene family are expanded indefinitely by using M-centered metal alloys to include C- and N-bonded transition metal species, such as Ta- [93] and Mo- [42] based MXenes, which are valued as reliable electrodes for supercapacitor applications. Conductivity, stability, and electrochemical outcomes are all significantly affected by the intrinsic properties of the M and X elements, and the surface finishes significantly affect these qualities as well. On the other hand, since their properties can be modified by options of different M and X components and surface terminals can be managed, there is tremendous potential to develop new MXene with favorable capacity properties. Therefore, considering their potential utility in supercapacitors, newer MXenes with higher electrochemical efficiency are needed. An appropriate synthesis approach, including etching and separation processes, should be optimized for each MAX precursor because it has a unique atomic bond. This is necessary to achieve the desired structure and achievement. There is a need for a large amount of research to investigate new MXene and preparation techniques, which represents an important and difficult task.
3. Heteroatoms Doping and the Control of Surface-Terminus Group
The surface properties and electron acceptability of the material can be changed by heteroatom doping. N-atom has been concluded to be an active heteroatom that affects the electrochemistry of MXenes, and many studies have examined these relationships. The reaction mechanisms can be summarized as follows: by replacing the C-atoms in the C-layer with N-atoms, it has been demonstrated that the distance between layers increases, effectively improving the electrical conductivity [94]. The most commonly used N-doping methods can be classified into heat treatment in ammonia [92], doping in liquid intermediates, water heat treatment [93,95], solvent heat treatment [96], and liquid peeling [97]. For example, as illustrated in Figure 14, a Ti3C2 film with a flexible N-doping layer was prepared by static adsorption and in situ solvent heat treatment using an alcohol solution saturated with urea as a source of N-atoms [96]. In addition, the intercalation of N in MXene increased the distanced of layers, therefore it offers the opportunity to tune the electrochemical properties.
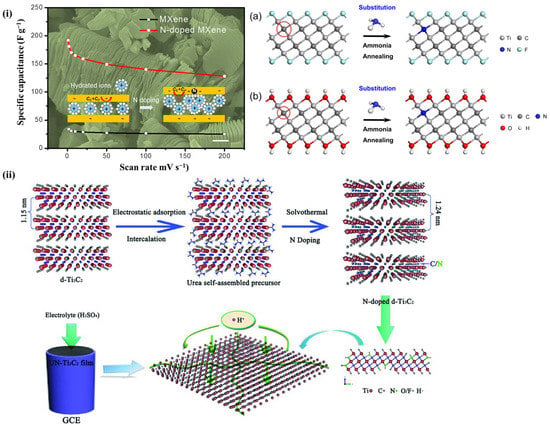
Figure 14. (i) An exemplification of charge storage and film-electrode fabrication of UN-Ti3C2 [96]. (ii) Schematic illustration of film preparation and electrode fabrication of the UN-Ti3C2.
Moreover, Ti2CTx-MXene underwent simultaneous liquid-gas phase delamination and chemical doping with nitrogen [97]. Under inert environs, the NH2CN utilized as a N-source and intercalant was bonded to the Ti2CTx nanosheets, and during heat treatment, a condensation reaction resulted in polymeric carbon nitride (p-C3N4). Solid-state doping was also shown to be possible. N and S co-doped Ti3C2Tx-MXene with a 75 F g−1 at 2 mV s−1 in Li2SO4 electrolyte were produced by pyrolysis of thiourea [98,99]. Nitrogen has been the primary focus of the majority of experiments investigating MXene doping. The potential to alter MXenes has also been demonstrated by using other types of doping atoms, such as metals and heteroatoms. The calculation results show that B-doping improves the stretchability of MXenes, thus making them ideal for use in pliable-electronics [100]. The interlayer spacing, conductivity, and capacitance of MXenes can also be increased by adding metal atoms such as V [101] and Nb [102] doping. As discussed above, chemical modification can be used to fine-tune the properties of MXenes and improve their outcome rate. As vacancies can lead to flaws in the MXene matrix and subsequently affect the structure, surface properties, and activity of MXenes, it may also be a useful strategy for modifying their properties. Novel types of terminus and doped atoms have been added to MXenes as a result of the growing depth of research examining chemical modification techniques [103,104,105,106,107].
4. Fabricating Vertical Alignments
The RGO/Ti3C2Tx MXene-modified fabrics were fabricated by a facile dip-coating and spray-coating method and the schematic illustration of the procedure is shown in Figure 15. The electrochemical performance of RGO and MXene-modified fabrics were characterized via CV tests in two-electrode configuration by using the 1 M H2SO4/PVA gel electrolyte. Figure 15a shows the CV curves of all-solid-state MCF and RMC supercapacitors at a scan rate of 10 mV s−1. The MCF-based supercapacitor shows the smallest enclosed CV curve (almost a line), indicating its inferior charge storage capability. Meanwhile, the charge storage capability was significantly increased after the incorporation of RGO, as the RMC-1 shows an enlarged CV area. In addition, the incorporation of RGO significantly enhanced the CA to 62.9 mF cm−2 for RMC-1, and the CA was further increased to 92.5 mF cm−2, 115.0 mF cm−2, and 258.0 mF cm−2 for RMC-2, RMC-3, and RMC-4, respectively. The increased CA was ascribed to the synergistic effects between the increased electrical conductivity and dominated pseudocapacitance of MXene. The topography images of the RGO/Ti3C2Tx MXene were analyzed by SEM and the obtained images are shown in Figure 15g,h. As shown in the SEM image, the RGO and MXene sheets are compactly filled in the fiber gaps and fiber surfaces, and graphene sheets can be obviously observed in the gaps between the weft yarns and warp yarns.
Efficient longitudinal ion transfer can be achieved by fabricating MXene membrane electrodes from “T-shaped” fragments [108]. The Ti3C2Tx film electrode in the H2SO4 electrolyte showed a capacity ranging from 2 A g−1 to 361 F g−1, and showed a capacity increase of 76% when the current density was increased to 20 A g−1. Table 2 summarizes the design and results of electrodes that can meet the capacity and current efficiency requirements of a supercapacitor. As illustrated in Table 2, the theoretical limit of MXene capacity can be approached by designing a hydrogel framework, while high-speed results can be obtained at a scan rate of up to 10 V s−1 using the porous MXene structure, and high capacity power with a film thickness of up to 200 μm can be obtained using vertically oriented MXene particles. This can confirm the output result. These designs and their combinations have provided new and exciting possibilities for MXene in supercapacitor applications.
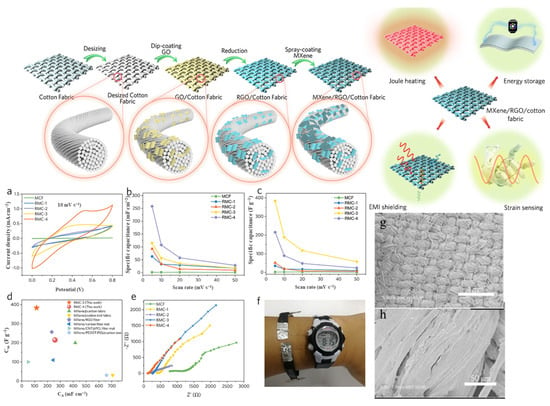
Figure 15. Schematic illustration of multifunctional RGO/Ti3C2Tx MXene fabrics, and electrochemical performance of the MCF and RMC fabrics. (a) CV curves of the MCF and RMC at 10 mV s−1. Areal specific capacitance (b) and gravimetric specific capacitance (c) of MCF and RMC. (d) Areal and gravimetric specific capacitance of RMC compared with the reported MXene-modified fibers, yarns, and fabrics. (e) Nyquist plots of MCF and RMC. (f) Four RMC-4 supercapacitors connected in series as a wristband to power a watch. (g,h) SEM images of fabrics [109].
Table 2. Capacitive outcome of MXene electrodes with optimized structural design.
| Electrode | Preparation | Electrolyte | Capacitance | Cyclability | Ref. |
|---|---|---|---|---|---|
| Controlling flake size | |||||
| Ti3C2Tx film | Mixing large and small flakes | 3 M H2SO4 | 435~86 F g−1 | 10,000 cycles | [69] |
| Adding spacer between MXene interlayer | |||||
| 75 μm Ti3C2Txpillared by hydrazine |
Suspending in hydrazine |
1 M H2SO4 | 250~210 F g−1 | no decay (10,000 cycles) |
[110] |
| Ti3C2Tx/graphene 3% film | Mixing and filtration | 3 M H2SO4 | 438~302 F g−1 | no decay | [111] |
| Sandwiched Ti3C2Tx/CNT 5% film |
Alternative filtration | 1 M MgSO4 | 390~280 F cm−3 | no decay | [112] |
| V2CTx/alkali metal cations film | Cation-driven assembly |
3 M H2SO4 | 1315~>300 F cm−3 | 106 cycles | [113] |
| Ti3C2Txionogel film | Immersing into EMITFSI | EMITFSI | 70~52.5 F g−1 | 1000 cycles | [114] |
| Ti3C2/CNTs film | Electrophoretic deposition |
6 M KOH | 134~55 F g−1 | no decay | [115] |
| carbon-intercalated Ti3C2Tx | In situ carbonization | 1 M H2SO4 | 364.3~193.3 F g−1 | 10,000 cycles | [116] |
| Ti3C2Tx@rGO film | Plasma exfoliation | PVA/H2SO4 | 54~35 mF cm−2 | 1000 cycles | [117] |
| Designing 3D/porous structure | |||||
| 13 μm Ti3C2Tx film | 1~2 μm PMMA template | 3 M H2SO4 | 310~100 F g−1 | - | [59] |
| MXene/CNTs film | Ice template | 3 M H2SO4 | 375~92 F g−1 | 10,000 cycles | [118] |
| Ti3C2 aerogel | assembly and freeze-drying |
1 M KOH | 87.1~66.7 F g−1 | 10,000 cycles | [119] |
| Ti3C2Txhydrogel | assembly and freeze-drying |
H2SO4 | 370~165 F g−1 | 10,000 cycles | [54] |
| Ti3C2Tx/carbon cloth | Freeze-drying with KOH treatment | 1 M H2SO4 | 312~200 mF cm−2 | 8000 cycles | [120] |
| Ti3C2Tx/Ni foam | Electrophoretic deposition | 1 M KOH | 140~110 F g−1 | 10,000 cycles | [121] |
| Compact and nanoporous Ti3C2Tx film |
Freeze-drying and mechanically pressing |
3 M H2SO4 | 932~462 F cm−3 | 5000 cycles | [122] |
| 3D porous Ti3C2Tx film | Reduced-repulsion freeze casting assembly |
3 M H2SO4 | 358.8~207.9 F g−1 | 10,000 cycles | [123] |
| Fabricating vertical alignments | |||||
| Anti-T Ti3C2Tx film | Filtrating through an entwined metal mesh |
1 M H2SO4 | 361~275 F g−1 | 10,000 cycles | [106] |
| 200 μm Ti3C2Tx film | Mechanical shearing of a discotic lamellar liquid-crystal MXene |
3 M H2SO4 | >200 F g−1 | 20,000 cycles | [105] |
5. 3D Microporous Sphere/Tube Ti3C2Tx
Due to strong van der Waals interactions, both V- and Ti-based MXenes tend to re-stack, resulting in decreased surface area and potassium ion kinetics, and many methods have been developed to fabricate 3D architectures using PICs in attempts to solve this problem. To generate 3D microporous spheres or tubes made of Ti3C2Tx, Fang et al. devised a facile spray-lyophilization process [124]. Figure 16a,b shows a 3D Ti3C2 structure consisting of several smooth-surfaced spheres and some tubes. The development of 3D morphology successfully solved the stacking problem of MXene nanosheets and enabled the possibility of fast ion transport (Figure 16c). The constructed PICs offered significant energy density and power density (98.4 Wh kg−1 and 7015.7 W kg−1). According to Figure 16d,e, Zhao et al. 3D K-pre-intercalated Ti3C2Tx (MXene) (K-Ti3C2Tx) was synthesized by electrostatic flocculation, freeze-drying, and KOH treatment, and the MXene interlayer gap escalated to 1.32 nm after K-intercalation, which was advantageous for the K+ kinetics. The proposed PICs exhibited an energy density of 163 Wh kg−1 and a high power density of 8.7 kW kg−1 with the combination of an AC cathode with a 3D K-Ti3C2Tx anode. In addition to the above study, the Zhang et al. research group fabricated a 3D foam-like 3D FMS by electrostatically neutralizing Ti3C2Tx with positively charged melamine followed by calcination [115], where the K conductivity was accelerated due to a surface area of 89.5 m2 g−1. Figure 16f shows the schematic illustration of the synthesis procedure of the 3D-FMS nanocomposites.
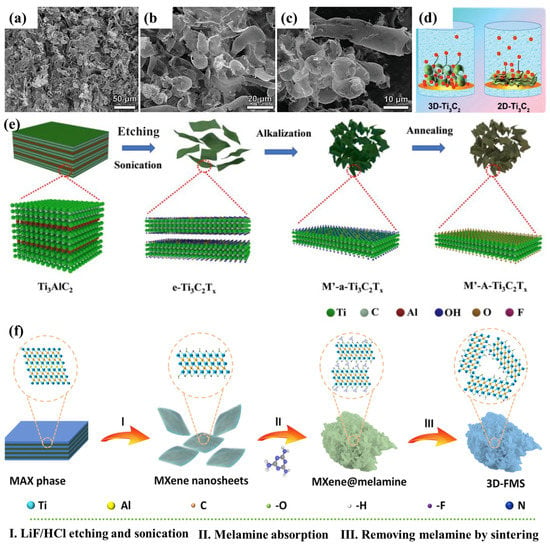
6. Design of 3D Porous MXene Electrode
By assembling 2D MXene wafers into a 3D electrode structure, the achievement of MXene materials for energy storage applications can be improved. The design of the MXene 3D foam was completed using a variety of technologies. Therefore, it is advantageous to reduce the size of the fragment and increase the interlayer distance so that the transport and accessibility of ions to the active site can be improved to increase the efficiency of MXene. However, by forming a 3D/porous electrode structure with large active surfaces that are accessible to ions as well as interconnected holes for ion-transport channels, the high throughput potential of MXene can be increased further. The achievement of MXene is expected to increase as appropriate porous structures can provide paths for electrolyte wetting and ion transport, thus reducing electrical resistance and shortening diffusion distance. Porous MXene electrodes have been generated by a variety of methods, such as chemical etching [33], template method [29], hydrazine-induced foaming [128], and electrical deposition [129]. Recently, small ice particles have been used as a self-sacrificing template for forming a flexible MXene/CNT film having large pores and nanopores. The controlled porous structure allows for capacities up to 375 and 251 F g−1 at 5 and 1000 mV s−1, respectively. Foaming, corrugation, and carbon assembly have also been suggested to be effective ways to create porous MXene frameworks [130,131,132,133].
It is also possible to combine 3D MXene aerogels with graphene [115]. As shown in Figure 17, both GO/MXene nanosheets are forced to gradually arrange along the ice crystal boundary during the lyophilization of the mixed GO and MXene solution, and they are eventually crosslinked through interactions to create a 3D lattice porous structure.
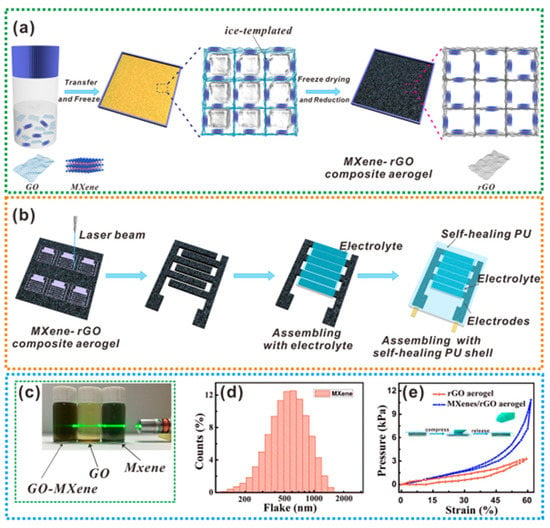
Figure 17. An exemplification of fabrication of (a) aerogel, (b) MSCs, (c) photo of NCs in dispersion of water, (d) size of MXene flake, and (e) pressure-strain curve [134].
This entry is adapted from the peer-reviewed paper 10.3390/nano13050919
This entry is offline, you can click here to edit this entry!
