Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Carcinoid tumors are a rare subtype of neuroendocrine tumor that arise in a variety of different organs and soft tissue. Explicitly, neuroendocrine tumors are characterized as neoplasms that are of neuroectodermal or epithelial origin and contain neurosecretory granules. Immunotherapy for the treatment of carcinoid tumors may still prove to be a useful treatment modality, particularly in combination with surgery and other pharmacologic regimens, such as somatostatin analogues.
- carcinoid
- immunotherapy
- biomarkers
1. Overview of Immunotherapy and Carcinoid
The rising interest in immunotherapy as a means of cancer therapy is rooted in its precision compared to the nonspecific approaches seen with surgery and chemotherapy. Active immunotherapy involves the direct stimulation of the immune system against the tumor, resulting in immunologic memory. It has been reported to have benefits in melanoma and lung cancer, as well as Hodgkin’s lymphoma [68]. Infusion of passive immunotherapies provide the host immune system with antibodies and cytokines that target cancer cells and elicit an immune response with limited duration. This form of therapy has previously demonstrated beneficial effects in metastatic melanoma [68]. Due to both therapies working within the immune system, they both have a wide range of cancers in which they can be utilized. Additionally, the restoration of the immune response seen with immunotherapy is able to increase the body’s ability to fight against tumor recurrence and metastasis, which numerous traditional therapies have difficulty protecting against [69]. On the other hand, there have been limitations in the use of immune therapies, primarily due to slow growth, low mutational burden, and the failure of carcinoid tumors to provoke a strong immune response [27].
2. Active Immunotherapy for Carcinoid
Active immunotherapy is the administration of specific agents to elicit a patient’s anti-tumor immune response [68]. These therapeutic agents include immune checkpoint inhibitors and oncolytic vaccines, which decrease the tumor cell’s ability to evade the cytotoxic effects of T cells [68]. This mechanism has shown to be beneficial in anti-tumor therapy, with efficacy in melanoma, lung, kidney and bladder cancers, while also increasing the proliferation of tumor-infiltrating lymphocytes. However, there are associated drawbacks that impose limitations on long-term treatment. In addition to high cost and unacceptable toxicities that may occur through activation of the immune system (also seen in passive immunotherapy), there have been issues regarding resistance producing negative regulation, resulting in autoimmune diseases and death [69].
2.1. Ipilimumab/Nivolumab
Ipilimumab, a humanized monoclonal anti-CTLA-4 antibody, was the first-in-class immune checkpoint inhibitor approved for the treatment of cancer [16]. In a prospective, open-label, multicenter phase II clinical trial (NCT02834013), the efficacy of ipilimumab plus nivolumab, a PD-1 inhibitor, is being evaluated in atypical bronchial carcinoid tumors, neuroendocrine carcinoma, and grade 3 NETs independent of the primary site [59]. In total, 32 eligible patients received the therapy, and of that, 18 patients had high-grade disease. The most common sites were gastrointestinal (n = 15) and lung (n = 6). The overall response rate (ORR) was 25% (CR 3% 1 pt, PR 22% 7 pts); those with neuroendocrine carcinoma demonstrated an ORR of 44% in patients with nonpancreatic high-grade neuroendocrine carcinoma, with 0% ORR in low/intermediate grade disease [59]. Seeking to validate these findings within the high-grade neuroendocrine neoplasm cohort, a second prospective study reporting ipilimumab plus nivolumab demonstrated a 26% ORR in patients with high-grade neuroendocrine neoplasms, with durable responses seen in patients with refractory disease [60]. The response rates can be compared to rates seen in randomized trials involving chemotherapeutic treatment of carcinoid tumors in Table 2. The combination of ipilimumab and nivolumab had limited to no efficacy for the treatment of carcinoid tumors. Current data suggest that the combination immunotherapy has a more prominent treatment role in metastatic neuroendocrine carcinomas.
2.2. Pembrolizumab
Pembrolizumab (Keytruda) is a humanized monoclonal anti-PD-1 antibody that has been broadly studied across a wide variety of neoplasms [70]. The Pembrolizumab with Lanreotide Depot for Gastroeneteropancreatic Neuroendocrine Tumors (PLANET) clinical trial (NCT03043664) investigated the effects of pembrolizumab with lanreotide, a somatostatin analogue with anti-tumor and serotonin suppression effects in patients with nonresectable, recurrent, or metastatic well-/moderately differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NETs). In total, 22 patients were treated—14 with gastrointestinal tumors and 8 with pancreatic tumors. Of the 12 GEP-NETs analyzed thus far, 4 contained datable PD-L1 expression. In this population, the combination of pembrolizumab and lanreotide achieved stable disease in approximately 40% of patients [62].
The KEYNOTE-028 study (NCT02054806) examined the efficacy and safety of pembrolizumab in biomarker-positive solid tumors, including 170 patients with PD-L1-positive advanced or metastatic carcinoid tumors, in addition to 106 patients with well- or moderately differentiated pancreatic NETs (pNETs). Of that, 21% of the well-differentiated pNETs and 25% of the moderately differentiated pNETs were PD-L1-positive tumors, being PD-L1+ carcinoid tumors, and 16 being PD-L1+ pNETs receiving treatment. For PD-L1 carcinoids receiving treatment, the ORR was 12% and 6.3% in the pNET group. Overall, this study shows that pembrolizumab demonstrates anti-tumor activity in a subset of NET patients [61].
The KEYNOTE-158 study (NCT02628067) investigated pembrolizumab in a larger cohort of patients, 1595 total participants, with advanced solid tumors. Included in this study is a total of 107 NET patients involving the lung, appendix, small intestine, colon, rectum, or pancreas who received treatment, 15.9% of whom had PD-L1-positive tumors. The ORR was 3.7%, demonstrating limited antitumor activity with pembrolizumab monotherapy [67].
2.3. Spartalizumab
Spartalizumab is a humanized monoclonal anti-PD1 antibody whose efficacy was studied in patients with well-differentiated metastatic NETs and poorly differentiated gastroenteropancreatic neuroendocrine carcinomas (GEP-NECs). The study (NCT02955069) included 95 patients with well-differentiated NETS and 21 in the GEP-NEC groups; the ORR was 7.4% in the NET group and 4.8% in the GEP-NEC group. Both groups fell below the predefined success criteria of ≥10%. However, it is worth mentioning that in those with thoracic NETs (n = 30), the ORR was 16.7%. Researchers concluded that the efficacy of spartalizumab is limited in this heterogenous group, although its efficacy with thoracic NETs warrants further investigation [71].
3. Passive Immunotherapy for Carcinoid
Passive immunotherapy is often referred to as adoptive cell transfer (ACT) or cell-based therapy. Rather than stimulating a patient’s immune system directly, as seen in active immunotherapy, passive immunotherapy focuses on extracting a patient’s lymphocytes (primarily T lymphocytes and natural killer cells) and altering them ex vivo, such that they become capable of attacking specific neoantigens. The cells are then reintroduced into the patient’s circulation, with the release of cytokines and cytolytic actions to destroy specific tumor cells [5]. Passive immunotherapy has been shown to have success in direct lymphocytic killing towards NETs based on various markers, such as SSTR2+ receptor, as well as multiple lymphocytic leukemias and lymphomas [5]. However, this form of therapy is more cumbersome in production compared to active immunotherapy due to its extraction–reinfusion process, which also decreases its reproducibility in targets, as well as in other patients.
3.1. Tidutamab
Tidutamab (Figure 3), previously known as XmAb18087, is a monoclonal bispecific antibody for SSTR2, a somatostatin receptor, and CD3 [66]. CD3 is an intracellular signaling domain in T cells that relays antigen engagement information from T-cell binding T-cell receptors [72]. The combination of the anti-SSTR2 and anti-CD3 activity of Tidutamab allows T-cell-mediated cytotoxicity directly towards SSTR2+ cells, often overexpressed in many NETs. In a phase I, multiple-dose, ascending-dose, escalation clinical trial (NCT03411915), the recommended dose, safety, efficacy, and anti-tumor activity of Tidutamab is being analyzed in 87 participants (42 at the time of this writing) with advanced NETs and gastrointestinal stromal tumors (GIST). Preliminary data as of August 2021 shows that Tidutamab is well-tolerated in solid tumors, with low incidence (41%) of cytokine-release syndrome, all recovered. There is a stable disease achieved in 26.8% of patients; however, a high PD-L1 clone E1L3N expression, especially seen in peripheral T cells, associates with poor treatment outcomes [66]. The association of high PD-L1 expression and poor treatment outcomes was unexpected. As mentioned earlier, monoanalyte biomarkers, such as PD-L1, can be inaccurate when predicting the response to immunotherapy. There are a multitude of PD-L1 clones, and their variation may lack comparability when assessing treatment outcome. Although Tidutamab can facilitate the recruitment of T cells and their binding to carcinoid tumor cells, the correlation of PD-L1 expression and poor treatment outcomes suggests that T cells are inactivated by PD-L1-positive tumor cells. The combination of Tidutamab and PD-1 or PD-L1 antibodies could result in a better treatment response.
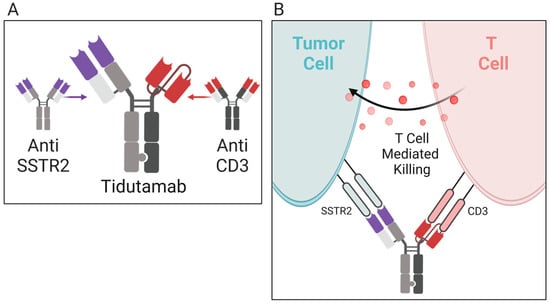
Figure 3. Mechanism of action for tidutamab. (A) Tidutamab is a bispecific antibody comprised of anti-somatostatin receptor (anti-SSTR2) and anti-CD3 found on T cells. (B) When activated, SSTR and CD3 trigger T-cell-mediated cytotoxicity. The tidutamab antibody combination facilities T-cell-mediated cytotoxicity against carcinoid tumor cells expressing the SSTR2 receptor.
3.2. 177Lu-Dotatate
Peptide receptor radionuclide therapy (PRRT) targets tumors using radioactive particles that bind specific receptors to deliver a localized dose of radiation. 177Lu-Dotatate is an example of PRRT, where a patient receives an injection of Octreotide/Octrotate (Dotatate) that is radiolabeled with lutetium-177. This therapy has been linked to treating NETs expressing somatostatin receptors. A phase III, multicenter, open-label clinical trial (NCT01578239), consisting of 229 patients with well-differentiated metastatic midgut NETs, is testing the safety and efficacy of 177Lu-Dotatate [63]. Primary analysis shows that after month 20, there is a 65.2% progression-free survival rate. Additionally, less than 10% of cases have reported clinically significant myelosuppression. The study demonstrated that 177Lu-Dotatate has great potential in treating metastatic GI NETs, while minimizing side effects typically seen with carcinoid therapies.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28052047
This entry is offline, you can click here to edit this entry!
