Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Cannabinoids are bioactive meroterpenoids comprising prenylated polyketide molecules that can modulate a wide range of physiological processes. Cannabinoids have been shown to possess various medical/therapeutic effects, such as anti-convulsive, anti-anxiety, anti-psychotic, antinausea, and anti-microbial properties.
- cannabinoids
- fungal platforms
- genetic engineering
1. Introduction
Cannabis sativa produces about 100 (alkyl-)phytocannabinoids (meroterpenoids and their analogs derived from cannabis), generically known as cannabinoids [1,2,3]. Cannabis is supposedly of Asian origin and is one of humanity’s oldest cultivated crops [4,5,6]. It is an annual, usually dioecious and sporadically monoecious, wind-pollinated species that is extremely allogamous (cross-fertilized) in nature [2]. The taxonomy of the Cannabis genus is complex due to the existence of cultivated and wild varieties, as well as its economic importance and uses (hemp, recreational or medical). The current botanical nomenclature [7] includes just one species in the genus, C. sativa, and two subspecies (subsp. sativa (fibers and oil cultivars) and subsp. indica (“narcotic” cultivars)) based on the THC (tetrahydrocannabinol) content (hemp varieties should not contain more than 0.3% THC in dried female flowering tops). The domestication status is also used as a characteristic to distinguish the two varieties within each subspecies [4,8,9].
The fiber varieties (“hemp”: fiber extracted from the stems of C. sativa) present a high carbon-sequestering potential due to their rapid growth, and so can be used as building materials or biofuel. However, the traditional use of fiber includes the manufacture of rope, shoes, canvas, paper, clothing, and sails [4,9,10]. On the other hand, the cannabinoids present in the sticky resin produced by the female plant, including psychoactive compounds such as THC and (−)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and non-psychoactive components with potential therapeutic effects (e.g., reducing inflammation, chronic pain, and nausea) such as cannabidiol (CBD) [11,12,13], are other relevant products of C. sativa.
2. Chemical Structure of Cannabinoids, Types, and Metabolic Routes for Biosynthesis
Phytocannabinoids are bioactive natural meroterpenoids with a resorcinol core bearing a para-positioned isoprenyl, alkyl, or aralkyl side chain [14]. The side chain usually contains an odd number of carbon atoms (cannabinoids containing an even number of carbon atoms are rare). Phytocannabinoids are found in angiosperms, fungi, and liverworts and are produced in several plant organs, such as the flower and glandular trichome of C. sativa, the scales of Rhododendron, and the oil bodies of Radula species. Moreover, it has been found that the mammalian brain has receptors that respond to compounds found in C. sativa. These cannabinoid receptors form the endocannabinoid system and regulate several biological functions and metabolic processes. Therefore, these bioactive compounds can be beneficial for the treatment of pain, anxiety, and cachexia in humans [15,16].
The different cannabinoids produced by C. sativa [17] are classified into structural families [18,19], such as cannabidiols (CBDs), cannabigerols (CBGs), cannabicyclols (CBLs), cannabinodiols (CBNDs), cannabinols (CBNs), cannabitriols (CBTs), cannabichromenes (CBCs), Δ9-THC, and miscellaneous cannabinoids [20]. The acid forms are the final product of the cannabinoid biosynthetic pathways, although several spontaneous modifications such as oxidation, decarboxylation, and cyclization frequently take place because of the poor oxidative stability of phytocannabinoids, in particular Δ9-THC [14].
Phytocannabinoids are accumulated in glandular thricomes all over the aerial parts of the plant, especially in the female flowers [21,22]. The phytocannabinoid biosynthetic pathway is split between different cellular compartments and organelles: the cytosol of gland cells (polyketide pathway), the plastids (methylerythritol 4-phosphate (MEP) pathway for prenylation), and the extracellular storage cavity (oxidocyclization and storage) (Figure 1). It is still unclear how intermediates and precursors are transported between compartments, although it is most likely that vesicle trafficking and transport proteins are involved in the movements of these intermediates across the interface between the gland cells and the storage cavity.
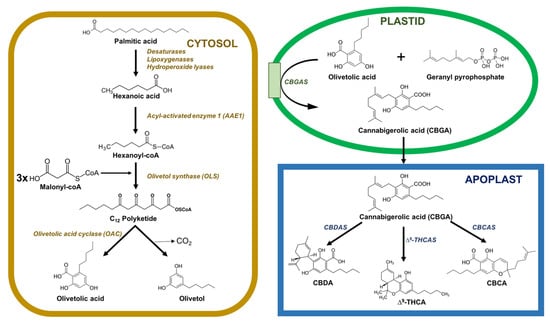
Figure 1. Subcellular distribution of enzymes catalyzing phytocannabinoid biosynthesis in C. sativa. Enzymes are located on the cytosol (yellow), plastids (green), or in the apoplastic space (blue). Abbreviations: CBCA, cannabichromenic acid; CBCAS, cannabichromenic acid synthase; CBDA, cannabidiolic acid; CBDAS, cannabidiolic acid synthase; CBGAS, cannabigerolic acid synthase; Δ9-THCA, Δ9-tetrahydrocannabinolic acid; Δ9-THCAS, Δ9-tetrahydrocannabinolic acid synthase.
Inside the cytosol, the biosynthesis of cannabinoids involves the integration of several steps in polyketide and isoprenoid metabolism. C18 fatty acids are desaturated, preoxygenated, and cleaved into hexanoic acid, which is transformed into thioester hexanoyl-CoA in a reaction catalyzed by acyl-activated enzyme 1 (AAE1). Later, the hexanoyl-CoA is elongated with malonyl-CoA in a reaction catalyzed by olivetol synthase (OLS) and cyclized by olivetolic acid cyclase (OAC) to produce olivetolic acid (OA) [23,24,25].
Inside plastids, geranyl pyrophosphate (GPP) is synthesized through the MEP pathway. OA is prenylated using GPP by cannabigerolic acid synthase (CBGAS), thereby forming cannabigerolic acid (CBGA), the first cannabinoid. CBGA is an essential cannabinoid because it is the precursor of several other cannabinoids. Then, the CBGA is converted into Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and cannabidiolic acid (CBDA) in the apoplastic space by the action of two enzymes, Δ9-THCA synthase (THCAS) and CBDA synthase (CBDAS), respectively. This conversion causes the reduction of O2 into hydrogen peroxide (H2O2) via oxidative cyclization reactions. Another important enzyme, cannabichromenic acid synthase (CBCAS), participates in the synthesis of cannabichromenic acid (CBCA) from CBGA using FAD and O2. THCAS, CBGAS, and CBDAS are also flavoproteins, which are strictly dependent on the presence of O2 as an electron acceptor. All these oxidocyclases carry a secretion signal peptide and are exported to the extracellular resin space. THCAS and CBDAS are active in the resin space, but it is still unknown whether their activity is exclusive to the extracellular space [23,26,27].
Δ9-THCA, CBDA, and CBDA with a pentyl side chain are the end-products of the enzymatic biosynthesis of cannabinoids and are synthesized in the apoplastic space. These active compounds undergo spontaneous rearrangement reactions when exposed to heat or radiation or during storage [23,26,28].
3. Importance, Applications, and Impact of Cannabinoids
Phytocannabinoids play several roles in human health, and cannabis preparations have been used in medicines since ancient times [29]. On the one hand, they exhibit anti-microbial activity against some bacteria and fungi, and they are effective against a wide range of infectious diseases in humans, acting as potent antibiotics. Cannabis extracts possess antimicrobial activity against some Gram-positive bacteria, such as Bacillus subtilis and Staphylococcus aureus, and the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. In contrast, no activity is displayed against Candida albicans and Aspergillus niger [30]. Phytocannabinoids (e.g., Δ9-THC, CBG, CBN, CBD, and CBC) display antibiotic activity against methicillin-resistant Staphylococcus aureus [31]. Δ9-THC and CBD exhibit bactericidal activity against streptococci and staphylococci but not against Gram-negative bacteria [32]. Because of their pharmacological potential, an increasing number of countries are relaxing their legislation around phytocannabinoids, and the global industry around cannabis-derived products is growing exponentially [33].
On the other hand, phytocannabinoids exert strong therapeutic potential in humans owing to their interaction with the G-protein-coupled cannabinoid receptors (GPCRs), such as CB1 and CB2; transient receptor potential (TRP) ion channels; and peroxisome proliferator-activated receptor (PPAR). CB1 is the most abundant GPCR in the central nervous system, and CB2 is located predominantly in the cells and tissues of the immune system [34]. Thus, cannabinoids play a key role in signaling, and the proper functioning of the immune and central nervous systems. Δ9-THC is the major psychoactive component of cannabis and displays pleiotropic effects in humans, including analgesic response, relaxation, pain tolerance, and dysphoria (anxiety disorder). Δ9-THC is also used by patients with insomnia and depression, since it improves sleep [35]. CBD exerts its function in humans through the CB1 and CB2 receptors in the CNS and the peripherical regions [36]. It is administered to patients with treatment-resistant epilepsy. CBD can also be delivered to patients receiving pharmacotherapy and can act as a potential cannabinoid to cure obesity, convulsive disorder, and rheumatoid arthritis. Furthermore, CBD also exhibits anti-psychotic, anti-nausea, and anti-anxiety properties [37].
In addition to the legal cannabis market (including medical and recreational uses), the EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) estimated the illicit cannabis market in 2017 to be around 1.4–1.7 tones, valued at EUR 10.5–12.8 billion [38]. Several clinical uses of cannabinoids have been described, but just a few are legally recognized by international regulatory agencies. The possible clinical applications of CBD have been summarized in sixteen diseases, which included several cancer types through anti-proliferative and anti-invasive action; inflammatory bowel and Chron’s diseases; cardiovascular diseases through anti-oxidant and anti-inflammatory properties; and neurodegenerative diseases (e.g., Parkinson’s and Alzheimer’s) [13]. However, in January 2020 the US Food and Drug Administration (FDA), FDA and Cannabis: Research and Drug Approval Process: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 1 February 2023) indicated that up to that date just one cannabis-derived drug product had been approved, Epidiolex, containing a purified form of CBD involved in the treatment of seizures associated with Lennox–Gastaut syndrome or Dravet syndrome in patients 2 years of age and older. In addition, three synthetic cannabis-related drug products have been validated by the FDA: (i) Marinol (dronabinol); (ii) Syndros (dronabinol); and (iii) Cesamet (nabilone). On the one hand, Marinol and Syndros represent a synthetic version of THC, the psychoactive component of cannabis, for the treatment of nausea due to cancer chemotherapy and anorexia, thus preventing weight loss in AIDS patients. On the other hand, Cesamet has a chemical synthetic structure similar to THC as an active ingredient, which is used to treat nausea associated with cancer chemotherapy.
Thus, in summary, cannabis as a hemp fiber is an economical reality, but the most promising market comprises the medical and recreative uses of the plant. However, the botanical derivatives are scarce in the legal market due to the putative risk of co-purification with other cannabinoids during the extraction process from naturally occurring plants. Nowadays, synthetic products are on the market through companies such as AbbVie Inc.; Corbus Pharmaceuticals Holdings Inc.; INSYS Therapeutics Inc.; and Bausch Health, although chemical synthesis is a costly process involving the use of chemicals that are not environmentally friendly. Nevertheless, synthetic production has paved the way for the biotechnological production of single cannabinoid compounds or derivatives in microorganisms, mainly fungi, which have been endowed with the biochemical machinery to reproduce the plant pathways and products [39], an approach that has been promoted by the development of recent synthetic biology methodologies [40].
This entry is adapted from the peer-reviewed paper 10.3390/jof9020234
This entry is offline, you can click here to edit this entry!
