Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Advancements in the fields of ionic liquids (ILs) broaden its applications not only in traditional use but also in different pharmaceutical and biomedical fields. Ionic liquids “Solutions for Your Success” have received a lot of interest from scientists due to a myriad of applications in the pharmaceutical industry for drug delivery systems as well as targeting different diseases. Solubility is a critical physicochemical property that determines the drug’s fate at the target site. Many promising drug candidates fail in various phases of drug research due to poor solubility.
- drug delivery systems
- ionic liquids
- water solubility
- cell permeability
1. Introduction
Ionic liquids (ILs) are, generally, salts containing poorly coordinated anions and cations at room temperature (RT) or below 100 °C. ILs are also known as ionic fluids, ionic melts, liquid electrolytes, fused salts, liquid salts, ionic glasses, designer solvents, green solvents, and solvents of the future [1][2]. Since 1914, when Paul Walden published the first article on ionic liquids (ILs) concerning ethylammonium nitrate, ILs have gained prominence due to their unique physiochemical characteristics [3]. Wilkes and Zaworotkoin 1992, discovered an imidazolium-based IL that is both air- and water-stable at ambient temperature, which expanded the field’s applicability beyond synthetic chemistry and its application in biomedical fields. ILs are green solvents employed in green technology, and other methods can also be used for green synthesis [4][5][6][7][8][9]. Early research endeavored to develop ILs as eco-friendly, nonvolatile, stable solvents and noncombustible solvents. Ionic liquids (ILs) belong to a class of materials that are composed of ions and have distinctive properties such as high thermal stability, high solvating power, and low vapor pressure. These features of ILs are very useful in a variety of applications, including pharmaceutical drug discovery. One of the main applications of ionic liquids in drug discovery is the development of new solvents for chemical synthesis. ILs can be used as solvents for various reactions, including organic synthesis, and can also be used to enhance the solubility of compounds in aqueous media, which then produce it easier to purify and isolate compounds. This is also important in the early stages of drug discovery [10].
Another application of ILs in drug discovery is developing new drug delivery systems. ILs can be used to create nanoparticles and micelle structures to deliver drugs to specific target sites. Additionally, it can be used to create solid dispersions, which can improve the bioavailability and solubility of the drugs [10].
Latest studies have widened the field and characterized ILs as salts having lower melting points at approximately 100 °C and an infinite range of optimizable properties, such as volatility, toxicity, instability, and flammability [11][12][13].
The ILs, as seen from the previously limited viewpoint as salts of imidazolium, pyrrolidinium, quaternary ammonium, phosphonium cations, or pyridinium, have expanded as additional cations, such as the bioinspired cations of guanidinium, cholinium and metal–based cations. They are combined with different anions to produce salts that satisfy the concept of ILs [14][15][16]. The emergence of more complex compounds supports the idea that the heterogeneity of the structure of an asymmetric sterical cation strongly interferes with both arranged packing inside a crystal lattice and the interaction with anions, which accounts for the compounds’ characteristically low melting point. Additionally, because of the expansion of the chemical space brought about by these recent developments, it is now possible to construct ILs with custom characteristics for both theoretical research and real-world applications. Application-driven studies of ILs are a thriving and expanding area of study with new horizons in therapeutics, particularly drug delivery (Figure 1A,B) [17].
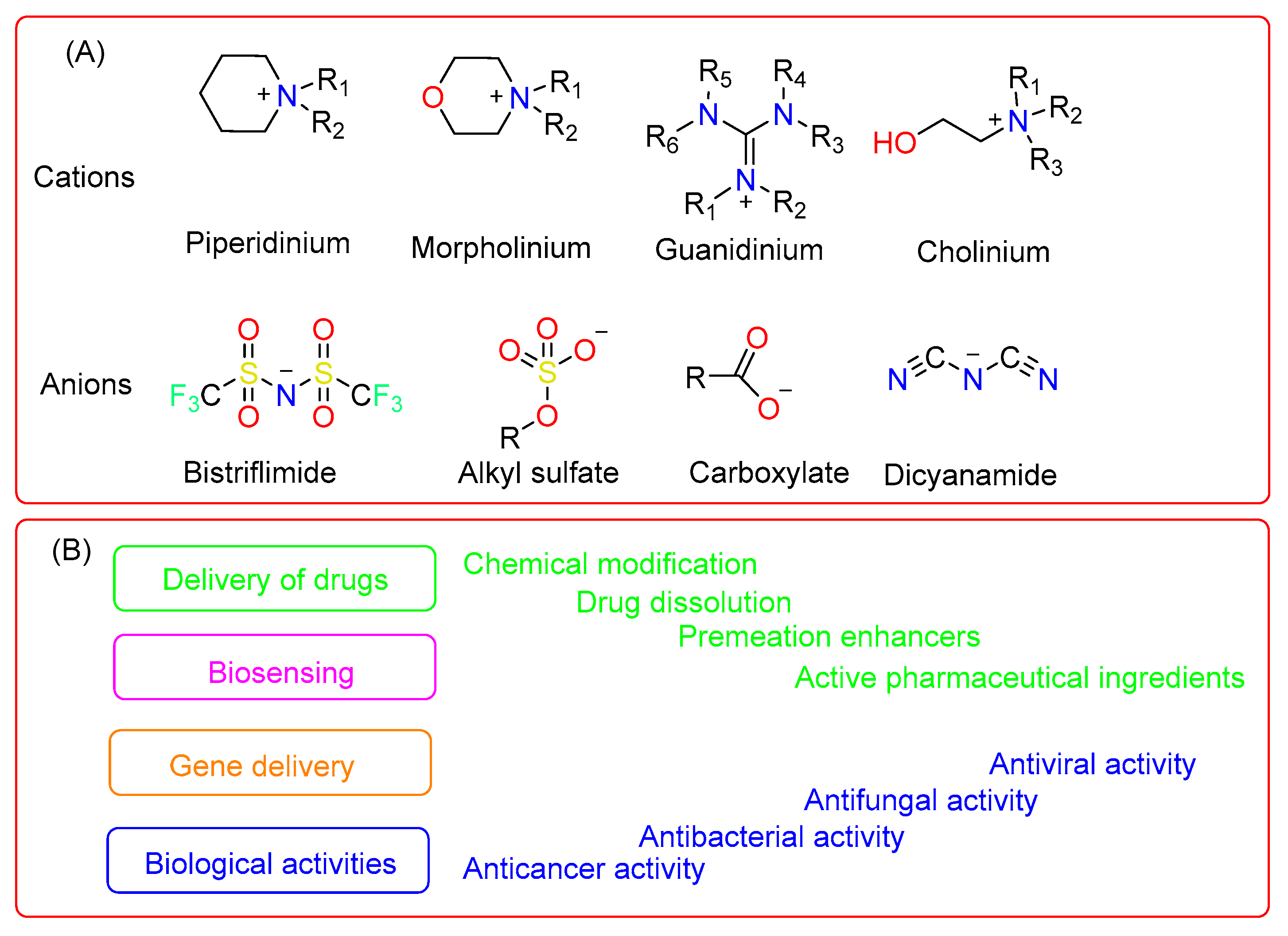
Figure 1. A list of different cationic and anionic molecules used in pharmaceuticals (A) and their various applications in different biomedical fields (B).
The poorly soluble profiles of many drug candidates limit their bioavailability and lead to clinical failure; hence, it is essential to develop innovative solubilization and formulation methods to address this issue. One of the robust approaches is the application of ILs as a drug delivery method [18][19][20]. Moreover, coupling a pharmaceutically active cation with IL-based compounds resulted in improved solubility that permits effective absorption via several barriers to reach target cells [21][22]. This combination is also known as active pharmaceutical ingredient ionic liquids (API-ILs).
2. Ionic Liquids (ILs) Used as Solvents
Organic unsafe solvents should be substituted with more environmentally friendly ones that are less volatile and flammable. ILs are particularly helpful in this condition since they often have very little vapor pressure [23][24]. Additionally, the careful selection of cations and anions may result in unique interactions with specific solute groups, which are crucial for the solubility of compounds with some complexity such as active pharmaceutical ingredients and their precursors [25].
2.1. Pharmaceutical Drug Synthesis Using Ionic Liquids as an Alternative Medium
Organic solvents are often used in the industrial synthesis of medicinal compounds, which causes organic contamination of the finished product and frequently contains leftover contaminants [26]. Compared to the reactions found in traditional organic solvents, those in ILs are often quicker and simpler to conduct, and they typically do not need any sophisticated equipment or methods [27]. Cations such as (C4MIM) paired with the (NTf2), (BF4), or (PF6) anions have been employed as media in the synthesis of APIs, regardless of the wide varieties of alternative cations and anions that are now accessible in the IL toolbox [9]. The synthesis of nucleoside-containing antiviral drugs such as stavudine, trifluridine, and brivudine using ILs as reaction media is well documented in the literature. For instance, the synthesis of trifluridine in IL media resulted in a higher yield of approximately 90–91% with less reaction time between 20–25 min [27].
The feasibility to recover and reuse of solvents was proven in some of the earliest cases. Zhang et al. used 1-butyl-3-methylimidazolium hexafluorophosphate ((C4MIM)(PF6)) in order to synthesize hybrids of pyrimidine nucleoside; thiazolini-4-one [28]. These hybrids have the potential to be used as antiparasitic therapeutics. Zunita et al. utilized (Emim) Cl as a reaction medium, to establish a green efficacious synthesis of 5-hydroxymethylfurfural (HMF) from monosaccharides. In this case, the solvent can be reproduced multiple times without losing efficacy [29].
In addition, ILs have been used in the manufacture of compounds that show remarkable promise in the treatment of cancer. ILs (C4MIM)(X) (where X = BF4 or PF6) were utilized by Wolan et al. [30] during the preparation of L-4- boronophenylalanine (L-BPA) (a clinically recognized substance) for the treatment of boron-neutron capture. The cross-coupling reaction after just 20 min was carried out with pinacol borane employing protected p-iodophenylalanine with (C4MIM)(BF4), this made it possible to synthesize L-BPA and its analogs with excellent yields ranging from 82–89%. Using a unique and effective biocatalytic approach, Kurata et al. were able to produce numerous analogs of caffeic acid phenethyl ester (CAPE) which showed antiproliferative activity. The researchers got a conversion yield of 92% by using Candida antarctica lipase B (Novozyme-435) in 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide as the medium. The same result has been found as CAPE was produced with isooctane [31].
Traditional nonsteroidal anti-inflammatory drugs (NSAIDs) have also been synthesized using ILs as an alternate medium. The pravadoline was synthesized in this scenario and was carried out with imidazolium-based ILs using a combination of Friedel–Crafts reaction and the nucleophilic displacement process [32]. With 1-butyl-2,3-dimethylimidazolium hexafluorophosphate, the alkylation of 2-methylindole with 1-(N-morpholino)-2-chloroethane was accomplished (99% yield). In the reactions that are carried out in ILs as a reaction medium, neither catalysts nor conditions that are strictly anhydrous are needed [33]. Some other NSAIDs (such as (S)-naproxen) were produced by employing precursors, Ru-BINAP catalyst and (C4MIM)(BF4), immobilized in IL; the resulting optical yields were comparable to those homogeneous reactions [34]. Monteiro et al. established widely viable lipases and two native lipases from Aspergillus terrus and Aspergillus niger, which were then tested to check the kinetic resolution of (R,S)-ibuprofen throughout the systems that contain (C4MIM)(PF6) and (C4MIM)(BF4) [34].
The use of ILs as substitute solvents for the production of various medicinal drugs is undoubtedly favorable. The applicability of ILs in place of organic hazardous solvents may often improve reaction conditions, speed up certain more difficult processes, and make it easier to purify and isolate the required product. From the perspective of the pharmaceutical industry, ILs may be a great solvent option for the production of certain particular drugs. The potential for IL recycling further expands the cost window for industrial-scale production of APIs in an IL environment [35].
2.2. Drug Delivery Using Ionic Liquids
It is possible to fine-tune the physicochemical characterization of ILs, which allows for efficient solutions to many issues associated with drug administration, such as limited drug insolubility and bioavailability. Researchers will discuss how ILs are now being used, specifically as solubility promoters, permeation enhancers, and API-ILs [36].
2.2.1. Enhancement of Permeability of Cell Membrane
The drug delivery systems are important factors in determining a drug’s effectiveness and must be developed in a way that prevents the drug from being subjected to unfavorable metabolism and transports it to the target at a speed that is acceptable [37]. However, there are several physiological barriers that prevent the drugs from being transported effectively. For instance, the stratum corneum (SC) impermeability, which presents a barrier to chemical absorption, continues to provide a challenge to topical and transdermal drug delivery, providing alternatives to injectable and oral methods (Table 2) (Figure 2) [38][39]. Therefore, a variety of methods such as chemical enhancers, are used to overcome the obstacles and assure effective drug delivery [40]. In this context, ILs have garnered interest and are now being researched more and more as chemical enhancers to promote trans- and para-cellular drug transport [41].
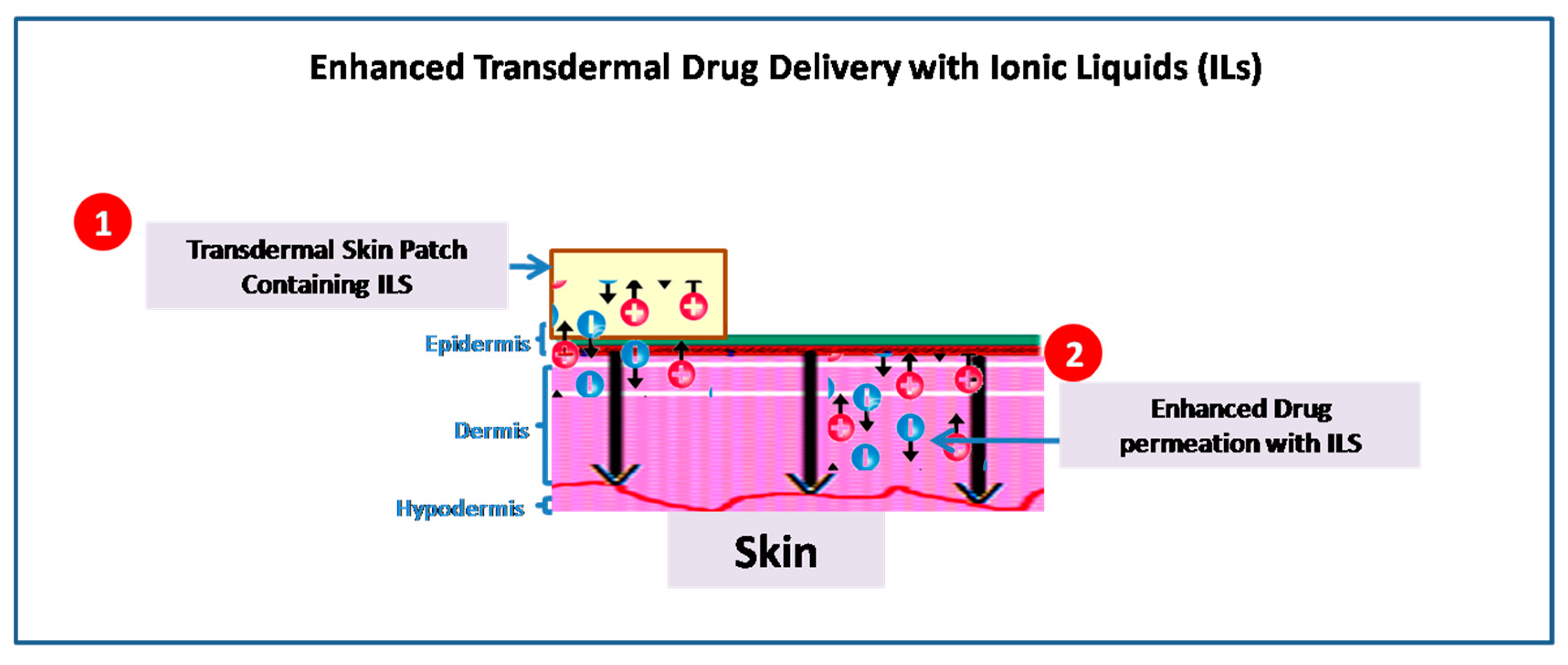
Figure 2. Illustration of permeation techniques of ILs applicable in transdermal drug delivery systems.
Indeed, the experimental evidence and computer models both support the viability of adopting ILs to improve drug delivery. An empirical force field and molecular dynamics, for instance, revealed that the amphiphilic nature containing 1-octyl-3-methylimidazolium-based IL with a cationic head has been used together in the model cell membrane, which causes compromised structural integrity and increases the permeability of ammonia through the membrane like small polar molecules [42][43][44]. The experimental evidence supported the hypothesis that the hydrophilicity of imidazolium-based ILs is responsible for the cell membrane solvation to open channels to facilitate molecular passage [44].
Although these discoveries are connected with cytotoxicity and the IL is responsible for enhancing the permeation power of the biological membranes, it is conceivable to anticipate that these processes should allow for the delivery of drugs to their intended targets. ILs are responsible for detaching lipids from physiological structures like the SC. Such lipid-targeted actions do produce flaws that improve drug permeability. Diversified IL-based enhancers work differently using various mechanistic ways to settle the functional and structural integrity of physiological barriers, which facilitate effective drug movements, provided that their physicochemical qualities let them do so. Hydrophilicity, for example, facilitates paracellular transport by opening the tight junction, while hydrophobicity favors entering the epithelial membrane via partitioning to promote transcellular transport [45][46]. The polar enhancers develop interaction and are then inserted inside the lipid and protein regions of the SC, starting to cause fluidization depending on concentration, whereas the non-polar enhancers primarily target the lipid to break down the barriers and develop the channels for molecules to carry out the diffusion process [47].
The indigenous toxicity of IL constructions motivates the investigation of the biomolecules as lead design towards the ILs’ next generation. The Cholinium cation, which is part of the cell membrane as a constituent of sphingomyelin and phosphatidylcholine and has been shown to readily permeate the cell membrane, is a developing bioinspired lead chemical. The coupling of cholinium cation with geranic acid (two equivalents) works as a pheromone in certain insects and also as a tyrosinase inhibitor in lemongrass [48]. Choline geranate (CAGE), which is a type 3 deep eutectic solvent (DES) for the delivery of drugs, is also an application of ILs [49]. DESs and ILs have low vapor pressure and high viscosity, but DESs are distinguished by their simple synthesis and a better understanding of the precursors’ toxicity. This study therefore examines CAGE and other DESs within ILs for the sake of simplicity. CAGE is a technique that serves as a chemical enhancer for transdermal distribution [16]. The capabilities of CAGE to deliver drugs are superior to those of controls, with the ability to boost the transport of mannitol and cefadroxil by 5 and 16-fold, respectively [50]. Other ILs such as tetraalkylphosphonium oleate, tetraalkylphosphonium hexanoate, choline oleate, and tetraalkylphosphoniumgeranate were developed as effective permeation enhancers, allowing a fivefold increase in the transport of cefadroxil through the dermis [50].
The property of molecules relies on the chemistry and structure of the IL, among other parameters. Indeed, the permeation of chemicals such as Transcutol® and ethanol are some examples, but in these instances, choline hexanoate and choline urea are the two typical examples of DESs, which inhibit the transdermal permeation of mannitol [50][51]. The CAGE is reported for traditional promoters for fluorescein isothiocyanate-marked insulin, OVA, and BSA more effectively than the typical enhancers. The CAGE is also reported for better insulin transdermal delivery in male Wister rats after the topical administration of 10 U/mL of insulin-CAGE combination [52].
Similar to CAGE, additional liquid salts generated from the carboxylic acid and aliphatic amines increase the drug’s penetration across physiological barriers. In a recent publication on the mode of action of this family of ILs, the Kubota group investigated a variety of compounds developed by reacting isostearic or octanoic acid using di- or tri-isopropanolamine [53]. A combination of aliphatic amine and acid in equal proportion produces ILs, which exist alongside unreacted amine and carboxylic acid. The unreacted amine and acid correlated to the permeability enhancement function of the ILs, which are good permeation enhancers for hydrophilic drugs but function as retarders for hydrophobic drugs. Such activity differs from the well-known chemical accelerator Azone, which easily transfers tulobuterol from the skin but is inefficient for phenol red, suggesting that the ILs have a different mode of action. Based on this observation, Kubota et al. hypothesized that ILs produced from aliphatic amines and acids can develop self-assembled nanoparticles, having polar head regions that created a hydrophilic core while the alkyl chain provides the hydrophobic corona [53]. The suggested model shows that the ILs have very distinctive boosting properties. Besides this, the hydrophilic core is responsible for wrapping the hydrophilic phenol red very efficiently as compared to the hydrophobic tulobuterol, whilst the hydrophobic corona acts as a permeant to transport the drug (which is encapsulated) across the hydrophobic nature-bearing SC. Although the production of nanoparticles has not been explicitly validated, the self-assembling properties of ILs in the form of nanostructures have been well-established [27].
2.2.2. Support for Low Solubility Drug Dissolution
ILs have a good solvating capacity, which increases the solubility of poorly soluble drugs, resulting in the enhancement of the absorption and the bioavailability of the drugs. The transdermal administration of IL-assisted acyclovir is one of the most important examples of solubility enhancement with ILs [36]. Shamshina and colleagues overcame this obstacle via the dissolution of acyclovir in hydrophilic ILs, which contains strong hydrogen bond acceptors such as dimethylphosphate and dimethylimidazolium to increase its solubility (>10%) [54].
As a result of the hydrophobic SC acting as an obstacle to the hydrophilic IL, Moniruzzaman and colleagues were unable to detect any drug diffusion occurring via the skin [55]. However, the diffusion was performed by developing an IL-in-oil (IL/oil)-based microemulsion with the IL phase that embodies acyclovir and the continuous oil phase, which overcomes the developed hydrophobic hindrance to convey the payload [55].
3. Ionic Liquid-Based New Active Pharmaceutical Ingredients
3.1. Pharmaceutical Salts Ionic Liquid (API-ILs) Synthesis
Generally, the synthetic procedure is a straightforward method to change the properties of the compounds using ionizable functional groups to eliminate undesired traits of the original drug. Prospective ions with poor symmetry and diffuse charge, which also describes several common APIs, are used in the choice of ion pairs to create ILs. Even the heterocycles with nitrogen that are now employed in ILs are typically found in APIs or their precursors. Nayl et al. reported the use of pyridinium-based dihydroxy ionic liquid ((Py-2OH) OAc) to improve the synthesis process and yield more potent anticancer compounds [56].
Numerous kinds of organic pharmaceutical salts have been established in the last century to alter the physicochemical or biological characteristics of compounds that was initially neutral. Figure 3 illustrates several organic pharmaceuticals that may be categorized as ILs. This group of pharmacological salt pairs—those that exhibit IL characteristics and comprise both anion and cation as active ingredients—has long been recognized in the literature. Examples of API-ILs include the antiseptic medicine cetylpyridinium chloride (melting point (m.p.) 77 °C, 1981), the anti-fibrillatory and antiarrhythmic drug bretylium (m.p. 86 °C, 1978), and the analgesic, anti-inflammatory, and antipyretic drug phenazone gentisate (m.p. 88 °C, 1951). Among examples of API-ILs, didecyldimethylammoniumibuprofenate, a local anesthetic and antiarrhythmic, lidocainium docusate, and ranitidine docusate, a histamine H2 receptor antagonist, were obtained using straightforward metathesis processes (Figure 3) [57].
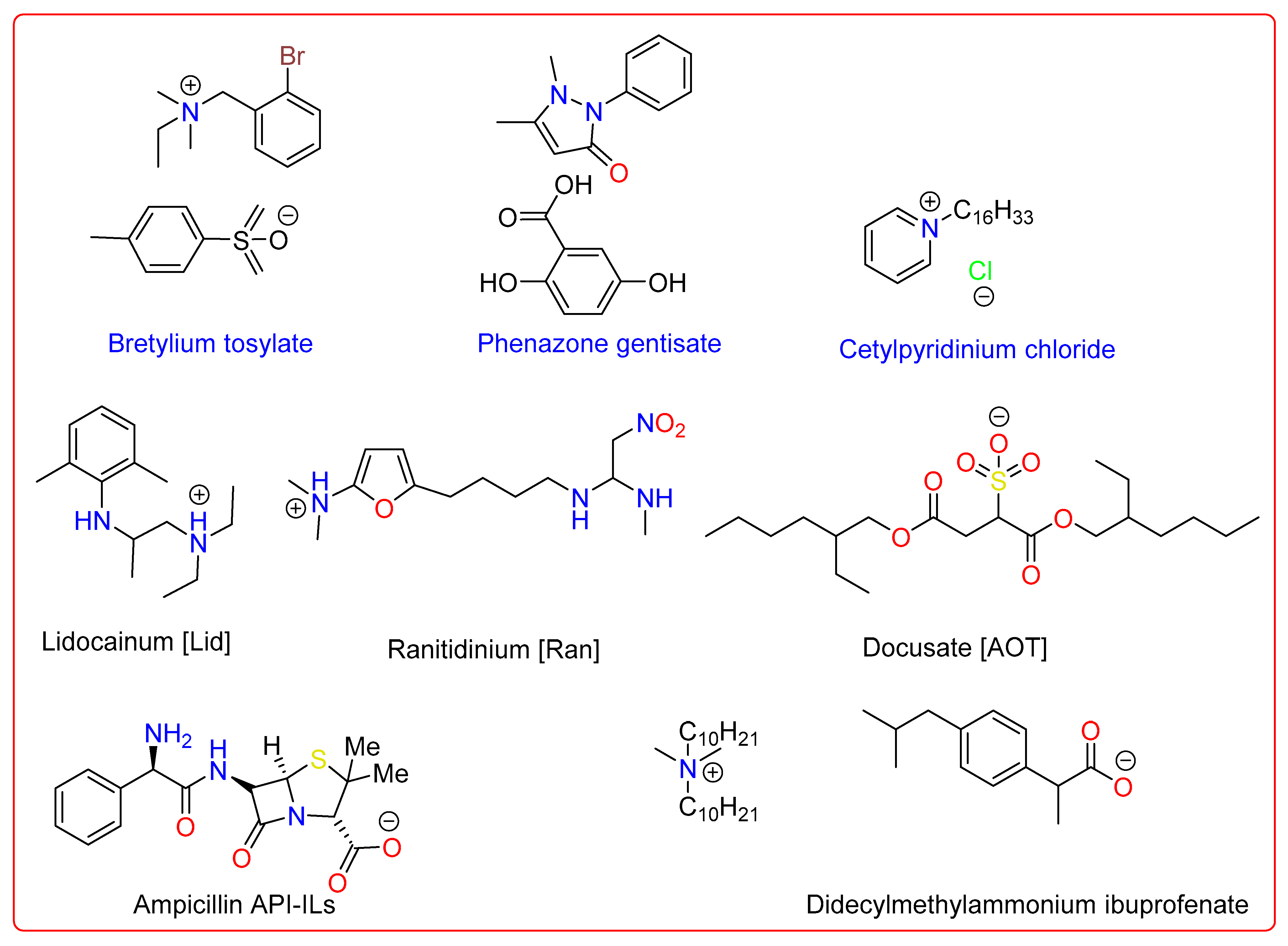
Figure 3. Examples of some active pharmaceutical ingredients-ionic liquids (API-ILs).
The majority of API-IL syntheses that have been documented in the literature include metathesis reactions. Individually dissolved in a chosen solvent (such as water, ethanol, methanol, or acetone), the cation and anion are in their commercially available salt forms. The solution is then agitated at an ambient temperature or heated to between 50 and 100 °C (if required). Because inorganic salts (such as NaCl) are present, this synthesis technology has certain limits in terms of the API-ILs’ ultimate purity [58]. However, these constraints may be overcome by selecting the right solvent or by utilizing additional purifying techniques. An extraction procedure using apolar solvents, such as chloroform or dichloromethane, is employed in API-IL purification whenever the inorganic salt is only the least part soluble in organic solvents. After that, the organic phase is washed with water to remove the inorganic salt (for example, NaCl, which can be verified by a silver nitrate test), and the solvent is eliminated using a rotary evaporator. The finished product is put on a high vacuum line in the last stage to get rid of any remaining solvent. In a few instances, further purification is mentioned, mostly to get rid of more halides [58].
The presence of residual contaminants significantly affects the physical, chemical, thermal, and biological characteristics of API-ILs produced via metathesis processes. The creation of innovative API-ILs depends heavily on the hunt for sustainable and more effective synthesis methods. ILs improve the physicochemical property and optimize the use of these substances. However, additional research is required to completely comprehend their effects on biological and pharmacological properties before they can be used on an industrial scale [59][60].
3.2. Salts of Protic Pharmaceuticals
Many significant APIs are deprotonated or protonated to create the frequently used salts with the appropriate pKa variations, rather than being permanent ions. The classification of protic ILs is a topic of significant discussion. In reality, drugs with modest levels of ionization offer many benefits over those that are completely ionized, most notably a better capacity to pass membranes [61]. 1-Methylhexylammonium salicylate is an example of a pharmaceutically useful, partly ionized IL. A nasal decongestant 1-methylhexylamine with 10.5 pKa value, and salicylic acid with 2.98 pKa value, were combined to develop a liquid with ambient temperature having the glass transition at 40 °C (pKa = 7.52) [61].
Nasal decongestants are made from tuaminoheptane, a primary amine base that has 10.50 pKa value. Amantadine is also a primary amine base with a pKa value of 10.10 (Figure 4). Nine out of the thirteen synthesized compounds meet the criteria for ILs having below 100 °C of melting points, with five of these compounds being ILs at ambient temperature. The high melting points are seen in all salts that include the amantadine base. All ((EtOH)PYRH)+-containing ILs have melting points under 100 °C, however, there are no clear patterns in the melting points of (NTH3)+-based ILs. To fully grasp the significance of protic ILs in therapeutic formulations, it is important to examine their chemical and thermal stability for each scenario [62].
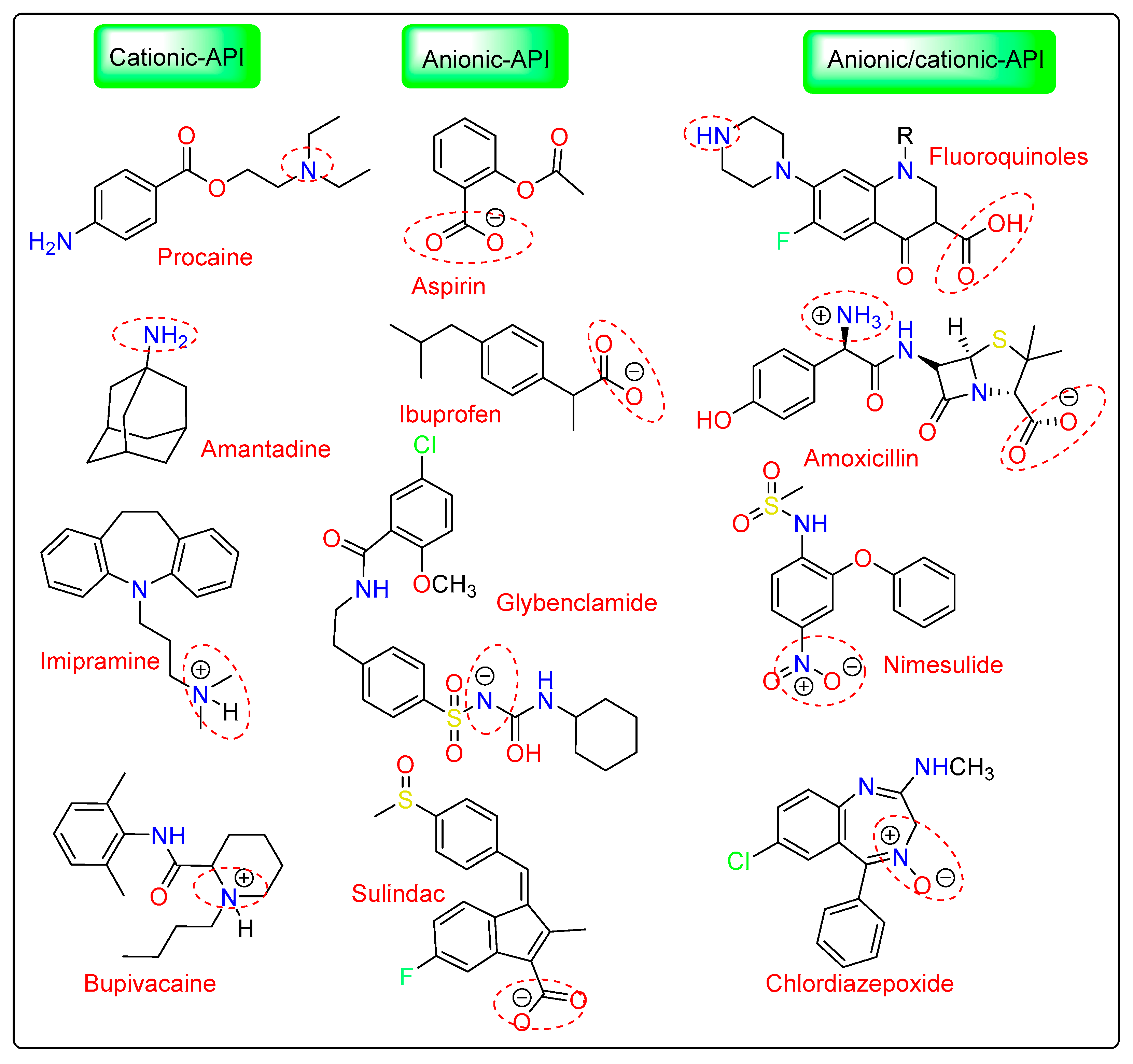
Figure 4. Some promising IL-assisted drugs with good solubilization and penetration properties reported for transdermal and topical modes of drug delivery.
3.3. Assessing Enhanced Properties of API-IL
Converting the active pharmaceutical ingredient (API) to ionic liquid is a tool to modulate the physicochemical properties such as solubility and stability of API. It is well-studied that most of the APIs change from amorphous to crystalline form during storage, which greatly affects their physicochemical properties and efficacy [21][22][62].
In this context, API-IL can be used to address the issue of polymorphic conversion. One of the examples is the improvement of the solubility of water-insoluble NSAID ibuprofen by using the (C2OHmim)+ cation. A combination of ibuprofen with on (C2OHmim)+ cation resulted in the solubility of ibuprofen being increased by 105 times [63]. Another application is the delivery of a combination of NSAIDs with local anesthetics. API-ILs have been used to increase the lipophilicity of topical NSAIDs with local anesthetics. API-ILs are an indisputable effective tool to produce liquid versions of therapeutic molecules [64]. The proton-transfer action of protic ILs (which is made up of active pharmaceutic anions) was explored by Stoimenovski et al. in model membrane transport [61]. In several publications, the stability of the ionic formulation of acidic drugs was considered troublesome. Despite the rapid growth of this theme, there is still more work to be done, especially in terms of evaluating the unique qualities of API-ILs [64][65].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020702
References
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635.
- Procopio, D.; Siciliano, C.; Trombino, S.; Dumitrescu, D.E.; Suciu, F.; Di Gioia, M.L. Green solvents for the formation of amide linkages. Org. Biomol. Chem. 2022, 20, 1137–1149.
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800, 405–422.
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967.
- Rao, R.V.; Patil, K.T.; Kumar, D.; Gupta, M.K.; Shin, D.-S. Mild and efficient one-pot synthesis of (E)-styrylperfluoroalkyl ketone from styrene. Results Chem. 2022, 4, 100260.
- Kharissova, O.V.; Kharisov, B.I.; Oliva, G.C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378.
- Liu, G.; Zhong, R.; Hu, R.; Zhang, F. Applications of ionic liquids in biomedicine. Biophys. Rev. Lett. 2012, 7, 121–134.
- Rao, V.R.; Patil, K.T.; Kumar, D.; Sebastian, S.; Gupta, M.K.; Shin, D.-S. Facile metal-free visible-light-mediated chlorotrifluoromethylation of terminal alkenes. Mon. Chem. 2022, 153, 495–500.
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426.
- Moshikur, R.M.; Goto, M. Ionic Liquids as Active Pharmaceutical Ingredients (APIs). In Application of Ionic Liquids in Drug Delivery; Springer: Singapore, 2021; pp. 13–33.
- Shi, W.; Luebke, D.R. Enhanced gas absorption in the ionic liquid 1-n-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) amide () confined in silica slit pores: A molecular simulation study. Langmuir 2013, 29, 5563–5572.
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709.
- Liu, Y.; Chen, J.; Li, D. Application and Perspective of Ionic Liquids on Rare Earths Green Separation. Sep. Sci. Technol. 2012, 47, 223–232.
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco (cyto) activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360.
- Dinis, T.B.V.; e Silva, F.A.; Sousa, F.; Freire, M.G. Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals. Materials 2021, 14, 6231.
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25.
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897.
- Zandu, S.K.; Chopra, H.; Singh, I. Ionic Liquids for Therapeutic and Drug Delivery Applications. Curr. Drug Res. Rev. 2020, 12, 26–41.
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51.
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active pharmaceutical ingredients (APIs) in ionic liquids: An effective approach for API physiochemical parameter optimization. Drug Discov. Today 2022, 27, 2415–2424.
- Ford, L.; Tay, E.; Nguyen, T.-H.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. API ionic liquids: Probing the effect of counterion structure on physical form and lipid solubility. RSC Adv. 2020, 10, 12788–12799.
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. J. Pharm. Sci. 2017, 106, 921–929.
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285.
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12.
- Pedro, S.N.; RFreire, C.S.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298.
- Kumar, V.; Malhotra, S.V. Synthesis of nucleoside-based antiviral drugs in ionic liquids. Bioorg. Med. Chem. Lett. 2008, 18, 5640–5642.
- Zhang, X.; Li, X.; Li, D.; Qu, G.; Wang, J.; Loiseau, P.; Fan, X. Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside-thiazolidinone hybrids and their antiparasitic activities. Bioorg. Med. Chem. Lett. 2009, 19, 6280–6283.
- Zunita, M.; Yuan, D.M.; Syafi’ Laksono, A. Glucose conversion into hydroxymethylfurfural via ionic liquid-based processes. Chem. Eng. J. Adv. 2022, 11, 100307.
- Wolan, A.; Zaidlewicz, M. Synthesis of arylboronates by the palladium catalysed cross-coupling reaction in ionic liquids. Org. Biomol. Chem. 2003, 1, 3274–3276.
- Kurata, A.; Kitamura, Y.; Irie, S.; Takemoto, S.; Akai, Y.; Hirota, Y.; Fujita, T.; Iwai, K.; Furusawa, M.; Kishimoto, N. Enzymatic synthesis of caffeic acid phenethyl ester analogues in ionic liquid. J. Biotechnol. 2010, 148, 133–138.
- Earle, M.J.; Seddon, K.R.; McCormac, P.B. The first high yield green route to a pharmaceutical in a room temperature ionic liquid. Green Chem. 2000, 2, 261–262.
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398.
- Monteiro, A.L.; Zinn, F.K.; de Souza, R.F.; Dupont, J. Asymmetric hydrogenation of 2-arylacrylic acids catalyzed by immobilized Ru-BINAP complex in 1-n-butyl-3-methylimidazolium tetrafluoroborate molten salt. Tetrahedron Asymmetry 1997, 8, 177–179.
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992.
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381.
- Carvalho, P.O.; Cass, Q.; Calafatti, S.A.; Contesini, F.; Bizaco, R. Review—Alternatives for the separation of drug enantiomers: Ibuprofen as a model compound. Braz. J. Chem. Eng. 2006, 23, 291–300.
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96.
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411.
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179.
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In silico screening using Molecular Dynamics simulations. Sci. Rep. 2019, 9, 1456.
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic liquid transdermal delivery system: Progress, prospects, and challenges. J. Mol. Liq. 2022, 351, 118643.
- Kumar, S.; Scheidt, H.A.; Kaur, N.; Kaur, A.; Kang, T.S.; Huster, D.; Mithu, V.S. Amphiphilic Ionic Liquid-Induced Membrane Permeabilization: Binding Is Not Enough. J. Phys. Chem. B 2018, 122, 6763–6770.
- Sindhu, A.; Bhakuni, K.; Sankaranarayanan, K.; Venkatesu, P. Implications of Imidazolium-Based Ionic Liquids as Refolding Additives for Urea-Induced Denatured Serum Albumins. ACS Sustain. Chem. Eng. 2020, 8, 604–612.
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818.
- Gao, L.; Lu, C.; Ma, S.; Yan, X.; Jiang, X.; Wu, X.; He, G. Flexibly crosslinked and post-morpholinium-functionalized poly (2, 6-dimethyl-1, 4-phenylene oxide) anion exchange membranes. Int. J. Hydrog. Energy 2020, 45, 29681–29689.
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Siahaan, T.J. Pathways and progress in improving drug delivery through the intestinal mucosa and blood–brain barriers. Ther. Deliv. 2014, 5, 1143–1163.
- Yu, A.S.L. Paracellular transport as a strategy for energy conservation by multicellular organisms? Tissue Barriers 2017, 5, e1301852.
- Boch, R.; Shearer, D.A. Identification of Nerolic and Geranic Acids in the Nassanoff Pheromone of the Honey Bee. Nature 1964, 202, 320–321.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379.
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144.
- Kubota, K.; Shibata, A.; Yamaguchi, T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur. J. Pharm. Sci. 2016, 86, 75–83.
- Shamshina, J.L.; Cojocaru, O.A.; Kelley, S.P.; Bica, K.; Wallace, S.P.; Gurau, G.; Rogers, R.D. Acyclovir as an ionic liquid cation or anion can improve aqueous solubility. ACS Omega 2017, 2, 3483–3493.
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250.
- Nayl, A.A.; Arafa, W.A.A.; Ahmed, I.M.; Abd-Elhamid, A.I.; El-Fakharany, E.M.; Abdelgawad, M.A.; Gomha, S.M.; Ibrahim, H.M.; Aly, A.A.; Bräse, S.; et al. Novel pyridinium based ionic liquid promoter for aqueous knoevenagel condensation: Green and efficient synthesis of new derivatives with their anticancer evaluation. Molecules 2022, 27, 2940.
- Wu, H.; Deng, Z.; Zhou, B.; Qi, M.; Hong, M.; Ren, G. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J. Mol. Liq. 2019, 283, 399–409.
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic liquids: Solvents and sorbents in sample preparation. J. Sep. Sci. 2018, 41, 209–235.
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207.
- Shamshina, J.L.; Berton, P.; Wang, H.; Zhou, X.; Gurau, G.; Rogers, R.D. Ionic liquids in pharmaceutical industry. In Green Techniques for Organic Synthesis and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 539–577.
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2010, 27, 521–526.
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776.
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911.
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids–challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215.
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E. Recent advances in ionic liquids in biomedicine. Adv. Sci. 2021, 8, 2004819.
This entry is offline, you can click here to edit this entry!
