Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Under optimized conditions, the designed sensing platform was found to sense IBP up to a 28 nM limit of detection. The interaction of ibuprofen (IBP) with bovine serum albumin (BSA) was investigated by differential pulse voltammetry. IBP−BSA binding parameters such as the binding constant and the stoichiometry of complexation were calculated. IBP and BSA form a single strong complex with a binding constant value of 8.7 × 1013.
- ibuprofen
- bovine serum albumin
- inflammation
1. Introduction
Drugs facilitate the prevention and cure of diseases by strengthening the immune system. The mechanism of drug action is a specific biochemical interaction that results in targeted pharmacological effect. This action includes binding of the drug molecule to a specific targeted biological species such as enzymes or receptors [1,2,3,4]. Overdosage of drugs can result in adverse short-term or long-term health effects [5]. Drugs affect or alter the physiology of living organisms [6]. They stimulate a biological reaction by targeting macromolecules in the body [7]. As a rule, most drugs impede a particular biological response by interfering with the neurological system (particularly the brain). Based on pharmacodynamics, drugs can be classified as depressants, hallucinogens, and stimulants. Drugs can also be classified into analgesics and therapeutics. The current work presents electroanalysis of a non-steroidal anti-inflammatory drug (NSAID), ibuprofen (Scheme 1), which is the third most popular, prescribed, and sold-over-the-counter drug in the world [8]. The World Health Organization has listed ibuprofen (IBP) as an “essential drug” [9]. It is extensively used as a pain reliever in conditions such as menstrual cramps, headaches, arthritis, and a wide variety of other common aches and pains [10,11]. It plays an anti-inflammatory role by prohibiting the production of pro-inflammatory prostaglandins through inhibition of the enzyme cyclooxygenase [12].
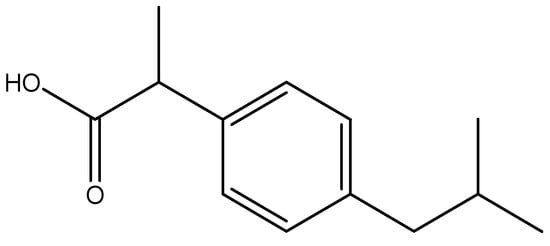
Scheme 1. Chemical structure of ibuprofen.
IBP enters into the environment due to improper pharmaceutical disposal during treatment. IBP manufacturing industries are a major contributor to the entrance of this drug into bodies of water. The toxicity of IBP metabolites exceeds that of the parent molecule. After excretion, IBP makes its way into wastewater treatment plants, sewage treatment plants, rivers, lakes, groundwater, soil, etc. A number of methods have been proposed for determining the concentration and effects of IBP in aquatic organisms [13]. To examine the short-term and long-term effects of IBP exposure, toxicity tests have been conducted on water-dwelling species. IBP is an emerging organic contaminant as its risk quotient is quite high [14]. Hence, part of the current work is focused on designing a sensitive electrochemical platform for the detection of IBP.
The protein–drug binding process involves complexation of a drug with protein. Protein–drug binding can be intracellular or extracellular. Intracellular binding involves the drug’s interaction with cell proteins, eliciting a pharmacological response; the receptors with which the drug interacts are known as primary receptors. Extracellular binding does not usually result in a pharmacological response, and such drug receptors are known as secondary or silent receptors. The nature of a drug binding to a protein can be reversible or irreversible [15]. IBP pharmacokinetically interacts with BSA, which is a natural and very abundant (59%) plasma protein. BSA has a high affinity for binding with drug ligands and metabolites [16]. As shown in Figure 1, BSA has three domains (I, II, and III) and two sub-domains (A and B). The predicted drug-binding site is present in the sub-domains II A and III A.
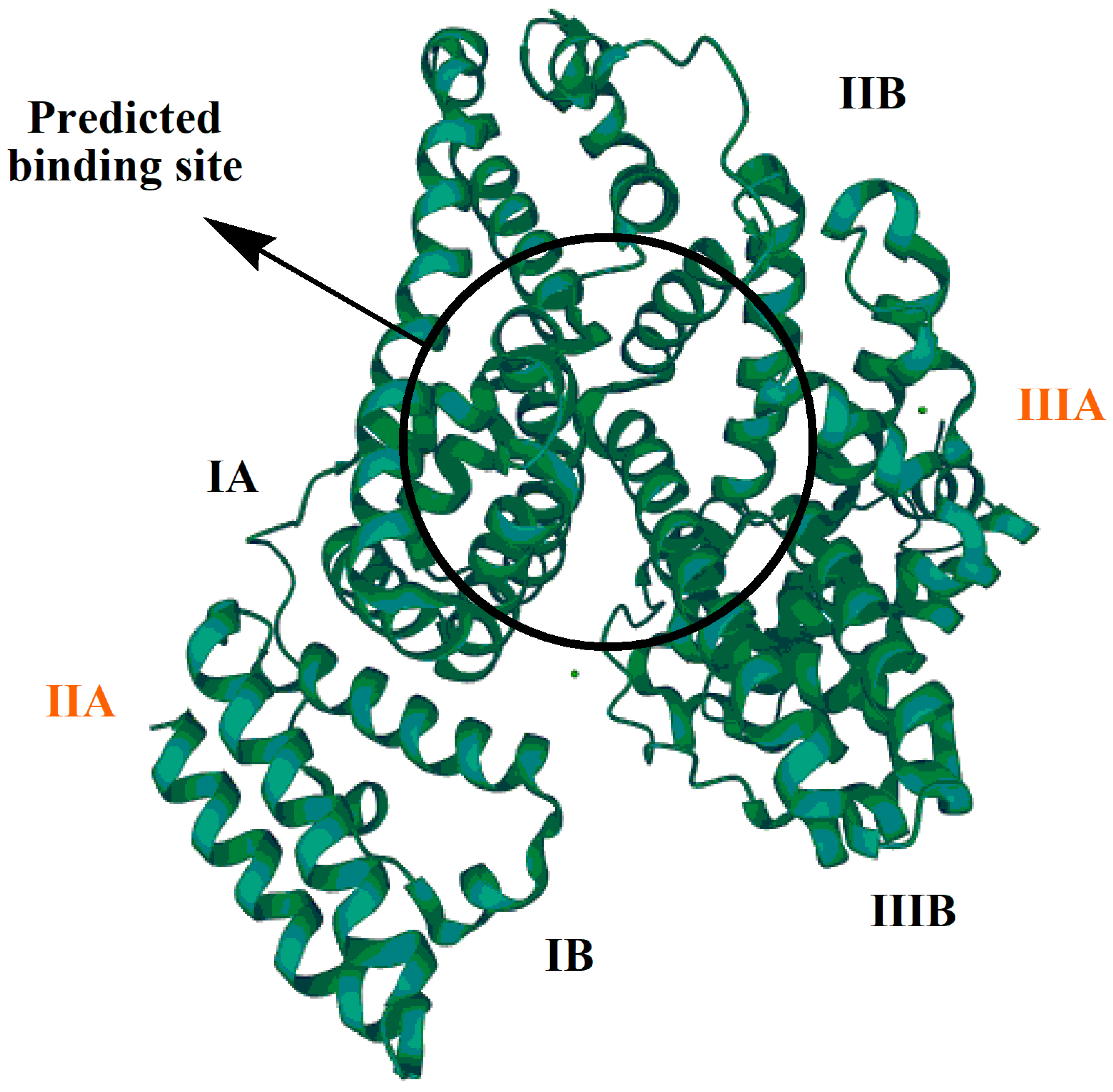
Figure 1. Bovine serum albumin (BSA).
Consider albumin (ALB) as the host protein that forms a complex with the guest drug (D). The binding constant (βs), also known as the affinity constant or association constant, is determined from the stoichiometry of complexation between ALB and D according to the following equation [17]:
Here ALB−mD represents the protein drug complex, and the stoichiometric co-efficient m indicates the number of drug molecules interacting per single molecule of protein. [FD] is the concentration of the free drug. The square brackets indicate the concentration of that particular species in Equation (2).
At equilibrium, βs can be obtained by:
Electrochemical study shows that by adding albumin to a constant volume of drug solution, the peak current of the drug decreases owing to its interaction with the albumin. The maximum decrease in peak current occurs when the maximum amount of a drug interacts with the protein. The decrease in peak current occurs due to slow mobility of the drug–protein complex as compared to the free drug. Hence, involvement of more drug molecules in interaction with proteins results in a smaller amount of the free drug concentration in solution. Moreover, a greater concentration of albumin leads to a maximum decrease in peak current intensity due to the formation of a greater number of complex molecules. Mathematically:
where K is the proportionality constant
As
By substituting the value of [ALB]total, one obtains the following equation:
The right side of the above equation is equal to βs[FD ]m according to Equation (2). Hence,
A linear relationship between Log(ΔIΔImax−ΔI) and Log [FD] indicates the formation of a single drug–protein complex. On the other hand, a non-linear relationship would suggest the formation of multiple complexes with different stoichiometry. If the Φ = Log(ΔIΔImax−ΔI) and Log [FD] plot shows two linear segments, then two types of complexes have been formed, and this will result in slopes m1 and m2. When multiple complexes are formed, then the following equation can be used:
where ΔI is the total decrease of peak current Ip obtained through a current voltage measurement.
All the above mentioned equations for binding constant determination involve calculation from peak current of the drug in the presence of protein. In this regard, electroanalytical techniques are the most promising options. The importance of the detection of drugs and their metabolites using an electrochemical platform for safeguarding the health of patients has pushed researchers to develop sophisticated electroanalytical tools for their monitoring [18]. The detection method should possess the qualities of high selectivity and sensitivity so that it can be effective for sensing biotoxins [19].
3. Electroanalysis of Ibuprofen and Its Interaction with Bovine Serum Albumin
A quick-responding, sensitive, and stable electrochemical sensor was developed using a composite of MWCNTs and Ag-doped ZnO nanoparticles as a modifier of a GCE surface. The designed sensor demonstrated excellent ability to detect IBP down to 28 nM. The peak current response of IBP was greatly improved by the components of the recognition layer in comparison to the bare GCE. Cyclic voltammetric and electrochemical impedance spectroscopic investigations revealed that the designed sensing scaffold has 4.5 times greater electroactive surface area and approximately 5 times less interfacial charge transfer resistance as compared to the bare GCE, which results in the generation of an intense signal for IBP oxidation. Voltammetric analysis revealed that the modified electrode possesses inter-day durability and four individually fabricated electrodes exhibited unaltered efficiency in terms of repeatability. The PG inhibition behavior of IBP was also investigated by measuring its binding capacity with BSA using DPV, where it was revealed that three molecules of IBP bind to a single molecule of BSA with a binding constant value of 8.7×1013. This strong binding potency of IBP suggests that it can play a significant role in PG inhibition and in turn can be developed as a medicine for reducing pain and inflammation. Considering the importance of IBP in the pharmaceutical industry and its medical potential, new and innovative analytical methods with high efficiency are still needed to effectively control this non-steroidal anti-inflammatory drug in pharmaceutical doses and to detect it in biological fluids. Moreover, coupling such sensors with industries for the early sensing of IBP in industrial effluents before their release to freshwater bodies may broaden their future applicability.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28010049
This entry is offline, you can click here to edit this entry!
