The natural flora of the mouth is very diverse. After the large intestine, it has the second highest complexity in terms of microorganisms, including more than 700 microorganisms. Each tissue has specific microbes that are different from adjacent tissues’ microbes. Still, these microbes can be displaced under certain conditions, such as the effects of cytotoxic drugs, oral cancer, or epithelial atrophy. The oral flora is divided into two categories, static and transient, which regularly balance with the host and protect against pathogenic microorganisms. The static flora on oral cavity surfaces is known as biofilm that can improve or protect oral health against pathogens, increase the virulence of potentially harmful microorganisms, and reduce the effectiveness of antimicrobial agents. Infections caused by bacteria in the mouth include caries and periodontitis. Microorganisms can attack different parts of the mouth via different mechanisms.
- oral infections
- orofacial infection
- orofacial microbes
1. Introduction
2. Herpes Simplex Virus
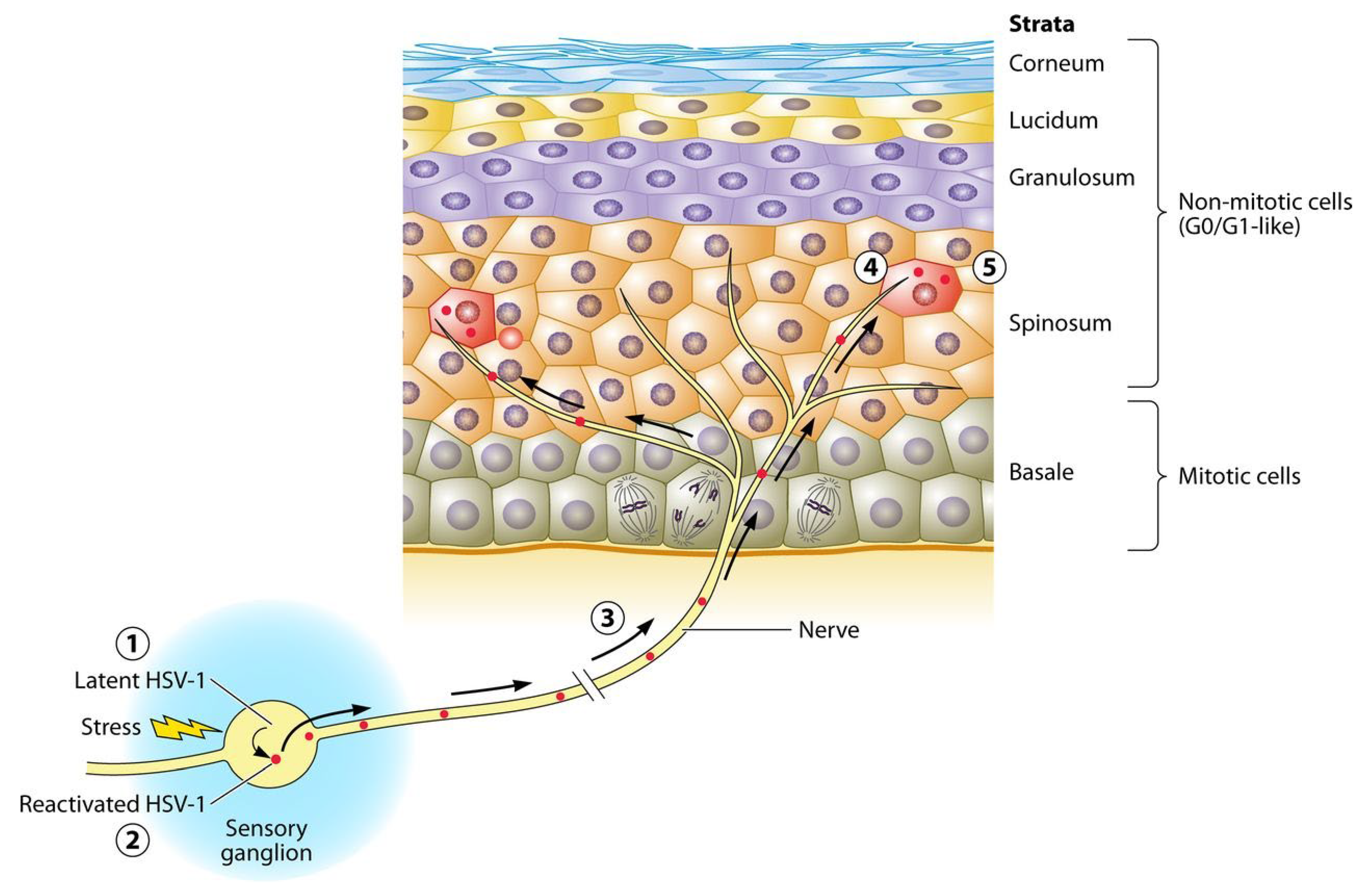
3. Human Papilloma Viruses
Papillomaviruses have a wide genetic diversity. Human papillomavirus (HPV) viruses use human cellular proteins to reproduce and survive [18]. The virus genome consists of open reading frames [19] and long control regions (LCR) to regulate the replication and transcription of primary genes [20]. The main reservoir of HPV is inflamed gums, salivary gland epithelium, and cryptal epithelium of tonsils, oral border, and oropharynx. The most clinically important genus of HPV is the alpha genus of human papillomavirus [18]. In high-risk HPV, placing the virus genome in the host genome breaks the virus genome at the E1 and E2 sites, and losing E2, in turn, causes E6 and E7 to lose control that, inhibits the regular function of p53 and pRb, respectively, and interfere with the cell cycle [21].
HPV infection can be transmitted from the mother’s cervix (sexual and non-sexual fomite transmission) and produces clinical or subclinical lesions. Oral HPV lesions include a range of benign oral lesions, lichen planus, fibroma, hyperplastic, papillomatous, verrucous, and carcinoma lesions. Generally, flat, exophytic, or wart-like white lesions in the oral mucosa, exophytic, wart-like, or papillary proliferations can be considered clinical manifestations of HPV [22]. Oral sex is the main transmission root in these diseases, and soft-circumscribed sessile nodular lesions and koilocytosis are some of their pathological and cellular manifestations [23]. The latent location of HPV in the mouth is usually in the gingival pocket because it is the only place where basal cells are in direct contact with the environment [24]. In about one-fourth of patients with periodontal disease in a clinical survey, the gingival samples have been associated with HPV [24].
There are several diagnostic methods for HPV. Immunohistochemical analysis-specific antibodies (e.g., p16INK4A and p16 IHC) and HPV mRNA/DNA-detecting PCR are the sensitive and cost-effective diagnostic methods for HNSCC tumor specimens. However, studies have shown that mRNA tests are the best approach for confirming the diagnosis [25]. Serological biomarker tests cannot be used for detecting HPV infection in the oral cavity. Their examination in oral fluids is useful for identifying and examining the incidence and course of the disease as they are low-cost, non-invasive, and local-specific [26].
Syrjänen discusses that HPV particles only get inactivated at temperatures 75–80 °C [27]. Preventive approaches such as vaccination and routine screening of HPV antibodies in the saliva are among the most effective ways to prevent HPV-associated head and neck diseases. Cold therapy, electrosurgery, surgical resection, laser therapy, and trichloroacetic acid are the usual treatments for papillomas/condyloma, verocroas, and FEH occurred by HPV [18].
4. Candida albicans
5. Aspergillus
6. Actinomyces
7. Streptococcus mutans
8. Streptococcus sanguinis
This entry is adapted from the peer-reviewed paper 10.3390/life13020269
References
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O’Brien, D. The Prevention of Infections in Older Adults: Oral Health. J. Am. Geriatr. Soc. 2020, 68, 411–416.
- Santosh, A.B.; Reddy, B.V. Oral Mucosal Infections: Insights into Specimen Collection and Medication Management. Dent. Clin. N. Am. 2017, 61, 283–304.
- Gopalakrishnan, U.; Murthy, R.T.; Felicita, A.S.; Alshehri, A.; Awadh, W.; Almalki, A.; Vinothkumar, T.S.; Baeshen, H.A.; Bhandi, S.; Kathir, A.; et al. Sulfate-Reducing Bacteria in Patients Undergoing Fixed Orthodontic Treatment. Int. Dent. J. 2022.
- Heboyan, A.; Avetisyan, A.; Skallevold, H.E.; Rokaya, D.; Marla, V.; Vardanyan, A. Occurrence of Recurrent Aphthous Stomatitis (RAS) as a Rare Oral Manifestation in a Patient with Gilbert’s Syndrome. Case Rep. Dent. 2021, 2021, 6648729.
- Heboyan, A.; Karobari, M.I.; Marya, A. Possible oral manifestation after vaccination against COVID-19: A case report. Oxf. Med. Case Rep. 2022, 2022, omac136.
- Heboyan, A.; Manrikyan, M.; Zafar, M.S.; Rokaya, D.; Nushikyan, R.; Vardanyan, I.; Vardanyan, A.; Khurshid, Z. Bacteriological Evaluation of Gingival Crevicular Fluid in Teeth Restored Using Fixed Dental Prostheses: An In Vivo Study. Int. J. Mol. Sci. 2021, 22, 5463.
- Abbasi, K.; Tavakolizadeh, S.; Hadi, A.; Hosseini, M.; Soufdoost, R.S.; Heboyan, A.; Alam, M.; Fani-Hanifeh, S. The wound healing effect of collagen/adipose-derived stem cells (ADSCs) hydrogel: In vivo study. Vet. Med. Sci. 2022; online ahead of print.
- Aurelius, E.; Franzen-Röhl, E.; Glimåker, M.; Akre, O.; Grillner, L.; Jorup-Rönström, C.; Studahl, M.; Group, H.-M.S. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: A double-blind, randomized controlled trial. Clin. Infect. Dis. 2012, 54, 1304–1313.
- Schang, L.M. Timing Is Everything. mBio 2018, 9, e02140-17.
- Whitley, R.J. Herpes Simplex Virus Infections of the Central Nervous System. Continuum 2015, 21, 1704–1713.
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochem. Biokhimiia 2014, 79, 1635–1652.
- Pires de Mello, C.P.; Bloom, D.C.; Paixão, I.C. Herpes simplex virus type-1: Replication, latency, reactivation and its antiviral targets. Antivir. Ther. 2016, 21, 277–286.
- Betz, D.; Fane, K. Herpetic Whitlow. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021.
- Damour, A.; Garcia, M.; Seneschal, J.; Lévêque, N.; Bodet, C. Eczema Herpeticum: Clinical and Pathophysiological Aspects. Clin. Rev. Allergy Immunol. 2020, 59, 1–18.
- Parra-Sánchez, M. Genital ulcers caused by herpes simplex virus. Enferm. Infecc. Y Microbiol. Clin. 2019, 37, 260–264.
- Balasubramaniam, R.; Kuperstein, A.S.; Stoopler, E.T. Update on oral herpes virus infections. Dent. Clin. N. Am. 2014, 58, 265–280.
- Samies, N.L.; James, S.H. Prevention and treatment of neonatal herpes simplex virus infection. Antivir. Res. 2020, 176, 104721.
- Syrjänen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 49–66.
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533.
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899.
- Kumaraswamy, K.L.; Vidhya, M. Human papilloma virus and oral infections: An update. J. Cancer Res. Ther. 2011, 7, 120–127.
- Lerman, M.A.; Almazrooa, S.; Lindeman, N.; Hall, D.; Villa, A.; Woo, S.B. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2017, 30, 1646–1654.
- Antonsson, A.; Cornford, M.; Perry, S.; Davis, M.; Dunne, M.P.; Whiteman, D.C. Prevalence and risk factors for oral HPV infection in young Australians. PLoS ONE 2014, 9, e91761.
- Anderson, K.S.; Wong, J.; D’Souza, G.; Riemer, A.B.; Lorch, J.; Haddad, R.; Pai, S.I.; Longtine, J.; McClean, M.; LaBaer, J.; et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br. J. Cancer 2011, 104, 1896–1905.
- Chai, R.C.; Lambie, D.; Verma, M.; Punyadeera, C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015, 4, 596–607.
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687.
- Shipilova, A.; Dayakar, M.M.; Gupta, D. High risk human papillomavirus in the periodontium: A case control study. J. Indian Soc. Periodontol. 2017, 21, 380–385.
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128.
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616.
- Seifi Kafshgari, H.; Yazdanian, M.; Ranjbar, R.; Tahmasebi, E.; Mirsaeed, S.; Tebyanian, H.; Ebrahimzadeh, M.A.; Goli, H.R. The effect of Citrullus colocynthis extracts on Streptococcus mutans, Candida albicans, normal gingival fibroblast and breast cancer cells. J. Biol. Res. 2019, 92, 8201.
- Reinhardt, L.; Nascente, P.; Ribeiro, J.; Guimarães, V.; Etges, A.; Lund, R. Sensitivity to antifungals by Candida spp samples isolated from cases of chronic atrophic candidiasis (CAC). Braz. J. Biol. 2020, 80, 266–272.
- Benito-Cruz, B.; Aranda-Romo, S.; López-Esqueda, F.J.; de la Rosa-García, E.; Rosas-Hernández, R.; Sánchez-Vargas, L.O. Oral Candida isolates and fluconazole susceptibility patterns in older Mexican women. Arch. Gerontol. Geriatr. 2016, 65, 204–210.
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312.
- Watthanasaen, S.; Merchant, A.T.; Luengpailin, S.; Chansamak, N.; Pisek, A.; Pitiphat, W. Xylitol-containing Chewing Gum for Caries Prevention in Students with Disabilities: A Randomised Trial. Oral Health Prev. Dent. 2017, 15, 519–527.
- Arya, N.R.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021.
- Tyring, S.K.; Lee, P.; Hill, G.T., Jr.; Silverfield, J.C.; Moore, A.Y.; Matkovits, T.; Sullivan-Bolyai, J. FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial. J. Med. Virol. 2017, 89, 1255–1264.
- Custodio, W.; Silva, W.J.; Paes Leme, A.F.; Cury, J.A.; Del Bel Cury, A.A. Plasma proteins in the acquired denture pellicle enhance substrate surface free energy and Candida albicans phospholipase and proteinase activities. J. Investig. Clin. Dent. 2015, 6, 273–281.
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106.
- Gacon, I.; Loster, J.E.; Wieczorek, A. Relationship between oral hygiene and fungal growth in patients: Users of an acrylic denture without signs of inflammatory process. Clin. Interv. Aging 2019, 14, 1297–1302.
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18.
- Obar, J.J. Sensing the threat posed by Aspergillus infection. Curr. Opin. Microbiol. 2020, 58, 47–55.
- Van De Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latge, J.-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661.
- Williams, C.; Rajendran, R.; Ramage, G. Aspergillus biofilms in human disease. Fungal Biofilms Relat. Infect. 2016, 931, 1–11.
- Rowe-Jones, J.M.; Moore-Gillon, V. Destructive noninvasive paranasal sinus aspergillosis: Component of a spectrum of disease. J. Otolaryngol. 1994, 23, 92–96.
- Chakrabarti, A.; Kaur, H. Allergic aspergillus rhinosinusitis. J. Fungi 2016, 2, 32.
- Telles, D.R.; Karki, N.; Marshall, M.W. Oral fungal infections: Diagnosis and management. Dent. Clin. 2017, 61, 319–349.
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. In Proceedings of Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 141–152.
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60.
- Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.-A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care 2015, 19, 7.
- Kawashima, J.; Nakajo, K.; Washio, J.; Mayanagi, G.; Shimauchi, H.; Takahashi, N. Fluoride-sensitivity of growth and acid production of oral Actinomyces: Comparison with oral Streptococcus. Microbiol. Immunol. 2013, 57, 797–804.
- Boyanova, L.; Kolarov, R.; Mateva, L.; Markovska, R.; Mitov, I. Actinomycosis: A frequently forgotten disease. Future Microbiol. 2015, 10, 613–628.
- Moghimi, M.; Salentijn, E.; Debets-Ossenkop, Y.; Karagozoglu, K.H.; Forouzanfar, T. Treatment of cervicofacial actinomycosis: A report of 19 cases and review of literature. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e627.
- Attaway, A.; Flynn, T. Actinomyces meyeri: From “lumpy jaw” to empyema. Infection 2013, 41, 1025–1027.
- Moturi, K.; Kaila, V. Cervicofacial actinomycosis and its management. Ann. Maxillofac. Surg. 2018, 8, 361.
- Balbinot, K.M.; Sousa, N.W.A.; Pinheiro, J.d.J.V.; Ribeiro, A.L.R. Surgical debridement as a treatment strategy for cervicofacial actinomycosis—Literature review and case report. Int. J. Surg. Case Rep. 2020, 73, 22–26.
- Lemos, J.A.; Quivey, R.G., Jr.; Koo, H.; Abranches, J. Streptococcus mutans: A new Gram-positive paradigm? Microbiology 2013, 159, 436.
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910.
- Yazdanian, M.; Rostamzadeh, P.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Moghaddam, M.M.; Kahnamoei, M.B. Evaluation of antimicrobial and cytotoxic effects of Echinacea and Arctium extracts and Zataria essential oil. AMB Express 2022, 12, 75.
- Strużycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135.
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29.
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 1–16.
- Braga, M.M.; Mendes, F.M.; Ekstrand, K.R. Detection activity assessment and diagnosis of dental caries lesions. Dent. Clin. 2010, 54, 479–493.
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569.
- Macey, R.; Walsh, T.; Riley, P.; Hogan, R.; Glenny, A.-M.; Worthington, H.V.; Clarkson, J.E.; Ricketts, D. Transillumination and optical coherence tomography for the detection and diagnosis of enamel caries. Cochrane Database Syst. Rev. 2021, 1, CD013855.
- Cochrane, N.; Walker, G.; Manton, D.; Reynolds, E. Comparison of quantitative light-induced fluorescence, digital photography and transverse microradiography for quantification of enamel remineralization. Aust. Dent. J. 2012, 57, 271–276.
- Frencken, J.; Innes, N.; Schwendicke, F. Managing Carious Lesions: Why Do We Need Consensus on Terminology and Clinical Recommendations on Carious Tissue Removal? SAGE Publications Sage CA: Los Angeles, CA, USA, 2016.
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018, 224, 219–225.
- Zeng, L.; Walker, A.R.; Lee, K.; Taylor, Z.A.; Burne, R.A. Spontaneous Mutants of Streptococcus sanguinis with Defects in the Glucose-Phosphotransferase System Show Enhanced Post-Exponential-Phase Fitness. J. Bacteriol. 2021, 203, e0037521.
- Li, Y.; Pan, Y.; Qi, F.; Caufield, P.W. Identification of Streptococcus sanguinis with a PCR-generated species-specific DNA probe. J. Clin. Microbiol. 2003, 41, 3481–3486.
