Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Compared with organic analogs, onium derivatives of nido-carborane have increased stability due to the stabilizing electron-donor action of the boron cage. Charge-compensated derivatives are considered according to the type of heteroatom bonded to a boron atom.
- nido-carborane
- charge compensation
- onium derivatives
1. Introduction
The synthesis of the first polyhedral boranes, carboranes, and metallacarboranes in the early 1960s was one of the major highlights in the development of inorganic chemistry over the last century [1]. The first reports on the synthesis of icosahedral carboranes appeared almost sixty years ago, at the end of 1963 when both the United States and the Soviet Union almost simultaneously declassified documents about their boron fuel projects [2,3,4,5,6]. A few months later, the nucleophile-promoted removal of one boron atom from the icosahedral ortho-carborane cage to form the 11-vertex nido-carborane cage species (Figure 1) was reported [7,8]. It was one of the most significant findings in the early years of the development of carborane chemistry, and now, more than five decades later, it remains indispensable for the synthesis of numerous metallacarboranes [9,10,11,12,13,14,15,16,17] and hydrophilic functionalized carboranes for medical [18,19,20,21,22,23,24,25,26,27] and other [28,29,30,31,32,33,34,35,36,37,38,39,40] applications.
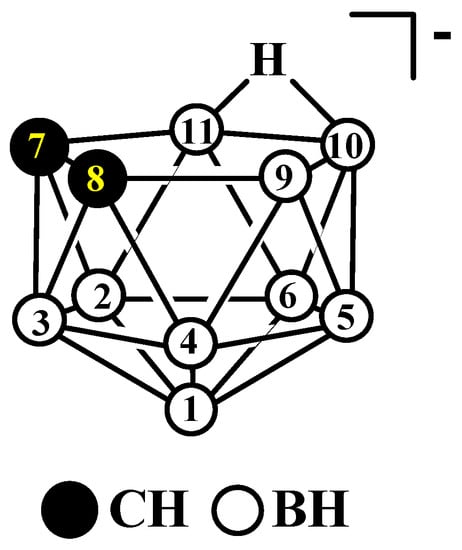
Figure 1. The idealized structure and atom numbering of nido-carborane [7,8-C2B9H12]−.
Metallacarboranes based on the dicarbollide ligand [7,8-C2B9H11]2−, which is formed upon the deprotonation of nido-carborane with strong bases, resemble the well-known transition metal cyclopentadienyl complexes. However, the dicarbollide ligand differs from the cyclopentadienyl ligand in a number of ways. In addition to its 3D character, the dicarbollide ligand is a significantly stronger donor than the cyclopentadienyl one and has a double charge. The donor nature of the dicarbollide ligand can be largely tuned via the introduction of substituents of various natures. At the same time, the charge of the ligand can be partially compensated by introducing into the dicarbollide ligand the so-called charge-compensating substituents of an onium nature (ammonium, phosphonium, sulfonium, etc.). This significantly brings the properties of the dicarbollide and cyclopentadienyl complexes closer together and causes a high interest in metallacarboranes based on charge-compensated dicarbollide ligands [41,42,43,44,45,46,47,48,49,50].
2. Charge-Compensated Derivatives of Nido-Carborane with Boron–Nitrogen Bond
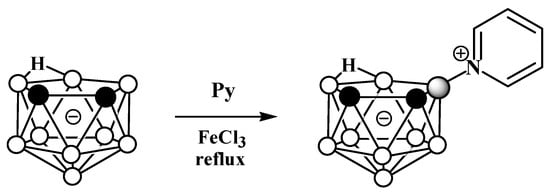
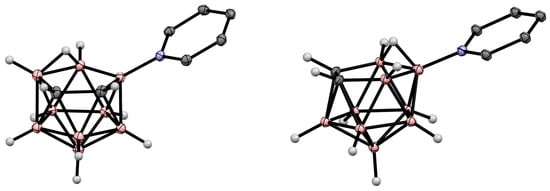
Due to the great diversity of nitrogen chemistry, the charge-compensated derivatives of nido-carborane with the B-N bond are characterized by the greatest variety of forms. The first example of the synthesis of charge-compensated derivatives of nido-carborane with a B-N bond was the reaction of the parent nido-carborane with pyridine in benzene in the presence of anhydrous FeCl3, leading to the asymmetrically substituted pyridinium derivative 9-Py-7,8-C2B9H11 (Scheme 1) [73], the structure of which was later supported via a single-crystal X-ray diffraction study (Figure 2) [74]. When FeCl3·6H2O was used instead of anhydrous FeCl3, the by-product of the reaction was the disubstituted pyridinium derivative 9,11-Py2-7,8-C2B9H9 [74]. The reaction with 7,8-dimethyl-nido-carborane proceeds in a similar way, giving 9-Py-7,8-Me2-7,8-C2B9H9 [73]. In a similar way, the reaction of nido-carborane with methyl isonicotinate in the presence of FeCl3 in refluxing benzene results in 9-(4′-MeO(O)CC5H3N)-7,8-C2B9H11 [75].

Scheme 1. The synthetic process to obtain 9-Py-7,8-C2B9H11.

Figure 2. Crystal molecular structures of 9-Py-7,8-C2B9H11 (left) and 10-Py-7,8-C2B9H11 (right). Hydrogen atoms of organic substituents are omitted for clarity.
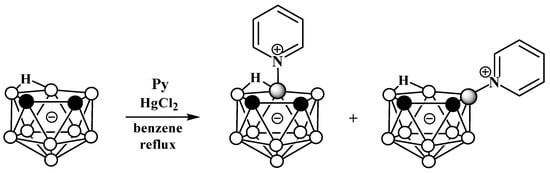
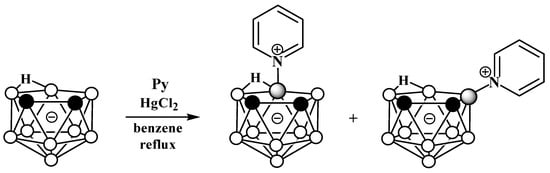
The reaction of nido-carborane with pyridine in the presence of HgCl2 in refluxing benzene gives a mixture of the symmetrically and asymmetrically substituted pyridinium derivatives 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 in a ratio of 2:1 (Scheme 2) [50,77]. The reaction of 7,8-dimethyl-nido-carborane [7,8-Me2-7,8-C2B9H10]− with pyridine proceeds in a similar way [77].

Scheme 2. Synthesis of 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 via the reaction of nido-carborane with pyridine in the presence of HgCl2.
The asymmetrically substituted 9-pyridinium derivative of nido-carborane was also prepared via the reaction of the parent ortho-carborane with pyridine in the presence of copper acetate and water. Similar reactions with C-monosubstituted ortho-carboranes give a mixture of the corresponding isomeric pyridinium derivatives 9-Py-7-R-7,8-C2B9H10 and 11-Py-7-R-7,8-C2B9H10 (R = Me, Ph). In the case of 1-XCH2 derivatives of ortho-carborane (X = Cl, Br, OH), in addition to a mixture of the corresponding 9- and 11-pyridinium derivatives of nido-carborane, the reaction gives the pyridinium methyl derivative 7-PyCH2-7,8-C2B9H11 [76].
The reaction of nido-carborane with pyridine in the presence of HgCl2 in refluxing benzene gives a mixture of the symmetrically and asymmetrically substituted pyridinium derivatives 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 in a ratio of 2:1 (Scheme 2) [50,77]. The reaction of 7,8-dimethyl-nido-carborane [7,8-Me2-7,8-C2B9H10]− with pyridine proceeds in a similar way [77].

Scheme 2. Synthesis of 10-Py-7,8-C2B9H11 and 9-Py-7,8-C2B9H11 via the reaction of nido-carborane with pyridine in the presence of HgCl2.
3. Charge-Compensated Derivatives of Nido-Carborane with Boron–Phosphorus Bond
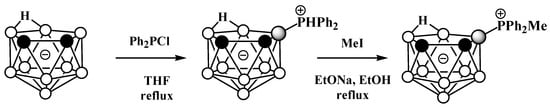
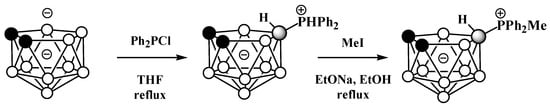
Unlike derivatives with a boron–nitrogen bond, derivatives of nido-carboranes with a boron–phosphorus bond can be prepared using electrophilic substitution reactions. Heating the potassium salt of nido-carborane K[7,8-C2B9H12] with Ph2PCl in tetrahydrofuran at reflux leads to the P-protonated diphenylphosphonium derivative 9-Ph2PH-7,8-C2B9H11, which can be alkylated with MeI under reflux in ethanol in the presence of EtONa as a base to give the methyldiphenylphosphonium derivative 9-MePh2P-7,8-C2B9H11 (Scheme 24, Figure 25) [103,104].

Scheme 24. The synthesis of 9-Ph2PH-7,8-C2B9H11 and its alkylation.

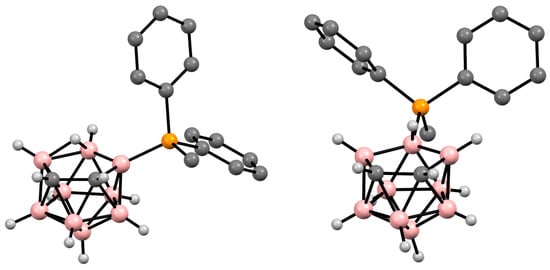
Figure 25. Crystal molecular structures of 9-MePh2P-7,8-C2B9H11 (left) and 10-MePh2P-7,8-C2B9H11 (right). Hydrogen atoms of phenyl and methyl groups are omitted for clarity.
The symmetrically substituted phosphonium derivatives 10-Ph2PH-7,8-C2B9H11 and 10-MePh2P-7,8-C2B9H11 (Figure 25) were prepared in a similar way using the disodium dicarbollide salt Na2[7,8-C2B9H11] as a starting material (Scheme 25) [104].

Scheme 25. The synthesis of 10-Ph2PH-7,8-C2B9H11 and its alkylation.
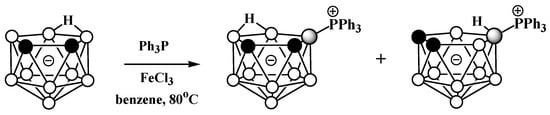
Phosphonium derivatives of nido-carborane also can be prepared via Lewis-acid-mediated nucleophilic substitution reactions. The reaction of the potassium salt of nido-carborane K[7,8-C2B9H12] with PPh3 in the presence FeCl3 in benzene at 80 °C leads to a mixture of triphenylphosphonium 9-Ph3P-7,8-C2B9H11 and 10-Ph3P-7,8-C2B9H11, which were separated using column chromatography on silica (Scheme 26) [103,104].

Scheme 26. Reaction of nido-carborane with triphenylphosphine in the presence of FeCl3.
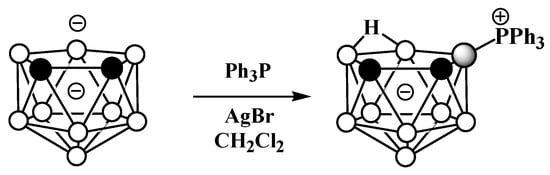
The asymmetrical triphenylphosphonium derivative 9-Ph3P-7,8-C2B9H11 was also obtained via the reaction of triphenylphosphine with the dithallium dicarbollide salt Tl2[7,8-C2B9H11] in dichloromethane and in the presence of AgBr at ambient temperature (Scheme 27) [105].

Scheme 27. Synthesis of 9-Ph3P-7,8-C2B9H11.
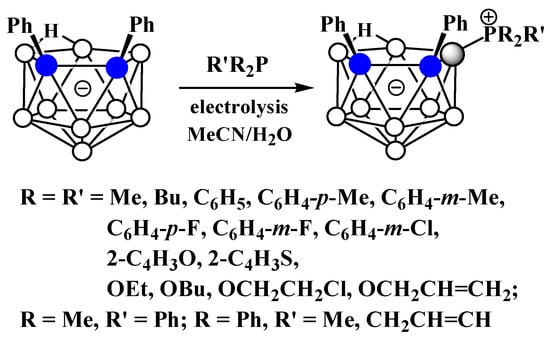
Similar to the pyridinium derivatives, a series of asymmetrically substituted phosphonium derivatives 9-R’R2P-7,8-Ph2-7,8-C2B9H9 was prepared via electrocatalyzed B-P oxidative couplings of 7,8-diphenyl-nido-carborane with various phosphines and phosphites (Scheme 28, Figure 26) [106].

Scheme 28. The electrocatalyzed B-P oxidative coupling of triarylphosphines, diarylalkylphosphines, and trialkylphosphines with 7,8-diphenyl-nido-carborane.

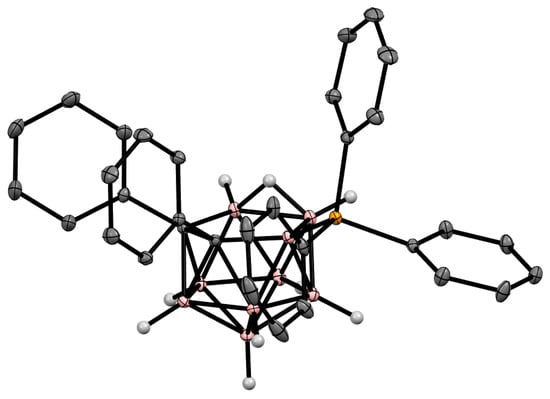
Figure 26. Crystal molecular structure of 9-Ph3P-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl rings are omitted for clarity.
4. Charge-Compensated Derivatives of Nido-Carborane with Boron–Arsenic and Boron–Antimony Bonds
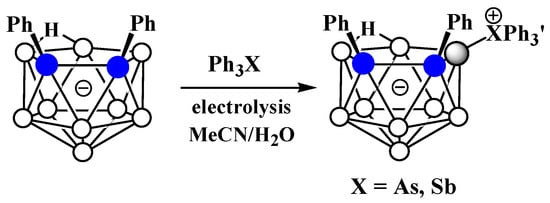
The charge-compensated derivatives of nido-carborane with boron–arsenic and boron–antimony bonds are rare and are limited to a few examples. Similar to the triphenylphosphonium derivative, the asymmetrically substituted triphenylarsonium and tetraphenylstilbonium derivatives 9-Ph3X-7,8-Ph2-7,8-C2B9H9 (X = As, Sb) were prepared via electrocatalyzed oxidative couplings of 7,8-diphenyl-nido-carborane with Ph3As and Ph3Sb, respectively (Scheme 44, Figure 36) [106].

Scheme 44. Synthesis of 9-Ph3As-7,8-Ph2-7,8-C2B9H9 and 9-Ph3As-7,8-Ph2-7,8-C2B9H9.

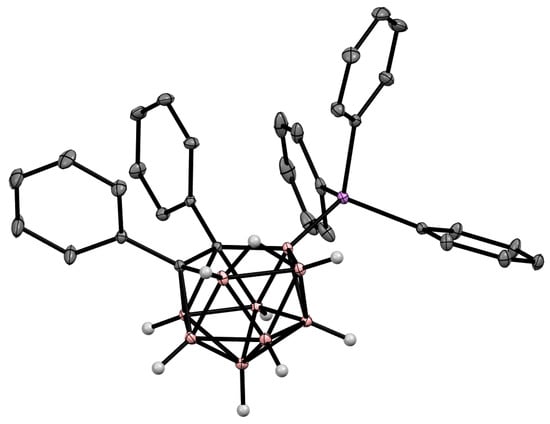
Figure 36. Crystal molecular structure of 9-Ph3As-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl groups are omitted for clarity.
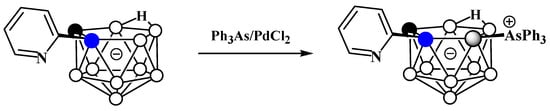
The reaction of 2-pyridyl-substituted nido-carborane [7-(2′-Py)-7,8-C2B9H11]− with triphenylarsine in the presence of catalytic amounts of PdCl2 in a mixture of toluene, water, and acetonitrile at 120°C results in the corresponding triphenylarsonium derivative 11-Ph3As-7-(2′-Py)-7,8-C2B9H10 (Scheme 45) [113].

Scheme 45. Synthesis of 11-Ph3As-7-(2′-Py)-7,8-C2B9H10.
5. Charge-Compensated Derivatives of Nido-Carborane with Boron–Oxygen Bond
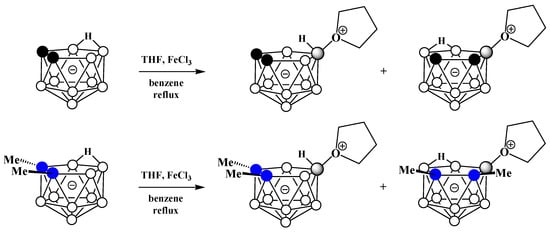
Alkyloxonium salts are much less stable than ammonium and phosphonium salts, and some of them are used in organic chemistry as strong alkylating agents. Nevertheless, strong electron-withdrawing of polyhedral boron hydride clusters substituted at boron atoms and, in particular, nido-carborane [117], is capable of stabilizing their oxonium derivatives [68,118]. The first example of such a derivative was obtained very soon after the discovery of nido-carborane via the reaction of the potassium salt of nido-carborane with tetrahydrofuran in the presence of FeCl3 in benzene. As a result, a mixture of two isomeric tetrahydrofuran derivatives of nido-carborane was obtained. The reaction with the C,C′-dimethyl derivative of nido-carborane proceeds in a similar way (Scheme 46) [73].

Scheme 46. Synthesis of 9-(CH2)4O-7,8-C2B9H11 and 10-(CH2)4O-7,8-C2B9H11 via the reaction of nido-carborane with FeCl3 in THF–benzene mixture.
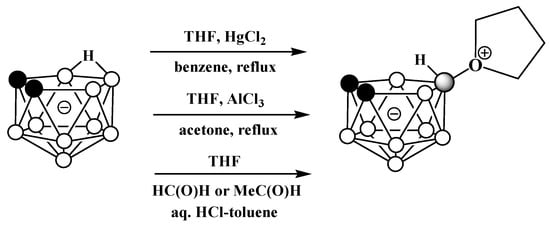
It was later found that the replacement of FeCl3 with HgCl2 in this reaction leads to the selective formation of the symmetrically substituted tetrahydrofuran derivative 10-(CH2)4O-7,8-C2B9H11 [77,119]. The symmetrically substituted derivative was also obtained via the reaction of the tetramethylammonium salt of nido-carborane with AlCl3 in a mixture of tetrahydrofuran and acetone [78] and by the treatment of the potassium salt of nido-carborane with tetrahydrofuran in the presence of acetaldehyde or formaldehyde and hydrochloric acid in a mixture of water and toluene [120] (Scheme 47).

Scheme 47. Different pathways for synthesis of 10-(CH2)4O-7,8-C2B9H11.
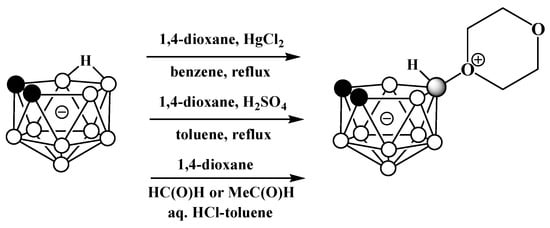
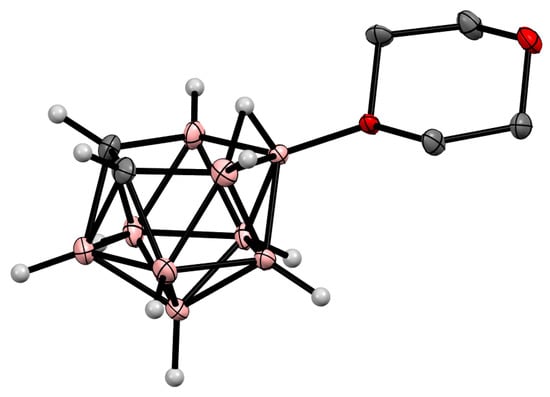
Oxonium derivatives of nido-carborane with other cyclic ethers were synthesized as well. The symmetrically substituted 1,4-dioxane derivative 10-O(CH2CH2)2O-7,8-C2B9H11 can be prepared via the reaction of the potassium salt of nido-carborane with 1,4-dioxane in the presence of HgCl2 in benzene [119] or in the presence of acetaldehyde and hydrochloric acid in a water–toluene mixture [120]. The 1,4-dioxane derivative can also be synthesized via the heating of the protonated form of nido-carborane 7,8-C2B9H13 with 1,4-dioxane [121] (Scheme 48). The molecular structure of the 1,4-dioxane derivative of nido-carborane was determined using single-crystal X-ray diffraction (Figure 37) [122].

Scheme 48. Different pathways for synthesis of 10-O(CH2CH2)2O-7,8-C2B9H11.

Figure 37. Crystal molecular structure of 10-O(CH2CH2)2O-7,8-C2B9H11. Hydrogen atoms of organic substituent are omitted for clarity.
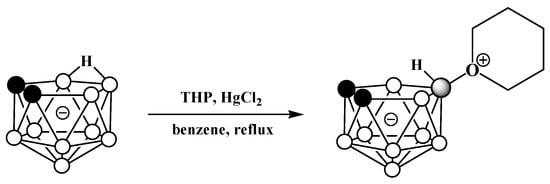
The reaction of the potassium salt of nido-carborane with tetrahydropyran in the presence of mercury(II) chloride in benzene results in the tetrahydropyran derivative 10-(CH2)5O-7,8-C2B9H11 (Scheme 49) [123,124].

Scheme 49. Different pathways for synthesis of 10-(CH2)5O-7,8-C2B9H11.
6. Charge-Compensated Derivatives of Nido-Carborane with Boron–Sulfur Bond
Compared with the oxonium derivatives, the sulfonium derivatives of nido-carborane are represented by a wider variety of derivatives and synthetic methods for their preparation. However, the most studied of them are the dimethylsulfonium derivatives of nido-carborane, which are widely used for the synthesis of metallacarboranes [41,50,135].
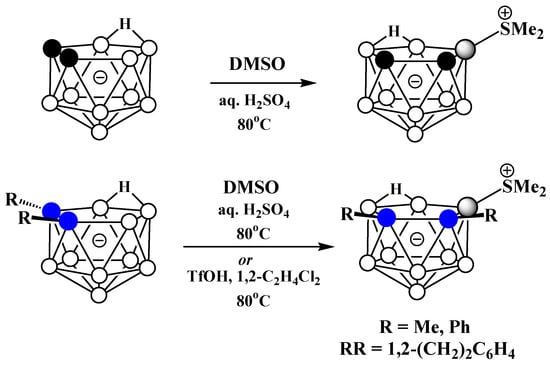
It should be noted that symmetrically and asymmetrically substituted dimethylsulfonium derivatives of nido-carborane are usually obtained in different ways, which excludes the formation of mixtures of their isomers. The asymmetrically substituted 9-dimethylsulfonium derivative of nido-carborane 9-Me2S-7,8-C2B9H11 was prepared via the reaction of the parent nido-carborane with dimethylsulfoxide in the presence of sulfuric acid at 80°C [74,136,137]. The reactions of the C,C’-substituted derivatives of nido-carborane proceed in a similar way, leading to the corresponding dimethylsulfonium derivatives 9-Me2S-7,8-R2-7,8-C2B9H9 (R = Me, Ph) [74,138] (Scheme 54). These conditions are similar to those used for the synthesis of the dimethylsulfonium derivatives of the closo-decaborate [139] and closo-dodecaborate [140] anions. The C,C′-substituted derivatives 9-Me2S-7,8-Me2-7,8-C2B9H9 and 9-Me2S-7,8-μ-(1′,2′-C6H4(CH2)2)-7,8-C2B9H9 were prepared via the reactions of the corresponding nido-carboranes with dimethylsulfoxide in the presence of triflic acid in 1,2-dichloroethane [134] (Scheme 54). The structures of 9-Me2S-7,8-R2-7,8-C2B9H9 (R = H, Ph) were determined using single-crystal X-ray diffraction (Figure 41) [141,142].

Scheme 54. Synthesis of the asymmetrically substituted dimethylsulfonium derivatives of nido-carborane 9-Me2S-7,8-R2-7,8-C2B9H9 (R = H, Me, Ph; RR = μ-1′,2′-C6H4(CH2)2).

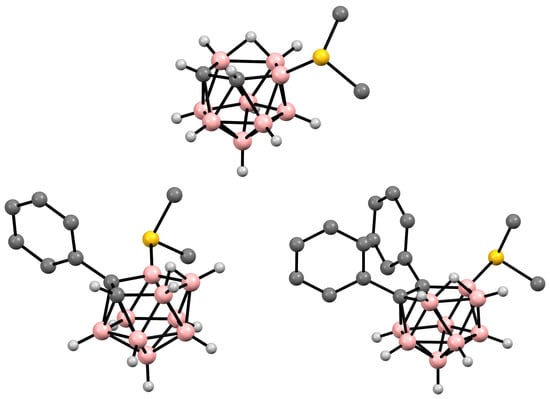
Figure 41. Crystal molecular structures of 9-Me2S-7,8-C2B9H11 (top), 11-Me2S-7-Ph-7,8-R2-7,8-C2B9H10 (bottom left), and 9-Me2S-7,8-Ph2-7,8-C2B9H9 (bottom right). Hydrogen atoms of organic substituent are omitted for clarity.
The asymmetrically substituted dimethylsulfonium derivatives 9-Me2S-7,8-Me2-7,8-C2B9H9 and 9-Me2S-7,8-µ-(CH2OCH2)-7,8-C2B9H9 were prepared via the reactions of the corresponding nido-carboranes with dimethylsulfide in the presence of Fe(NO3)3 in aqueous ethanol [49].
In the case of C-substituted nido-carboranes, such as K[7-Ph-7,8-C2B9H11], the introduction of a Me2S group results in a mixture of 9-Me2S-7-Ph-7,8-C2B9H10 and 11-Me2S-7-Ph-7,8-C2B9H10 isomers, which can be separated using column chromatography [137]. The molecular structure of 11-Me2S-7-Ph-7,8-C2B9H10 was determined using single-crystal X-ray diffraction (Figure 41) [138].
7. Charge-Compensated Derivatives of Nido-Carborane with Boron–Selenium and Boron–Tellurium Bonds
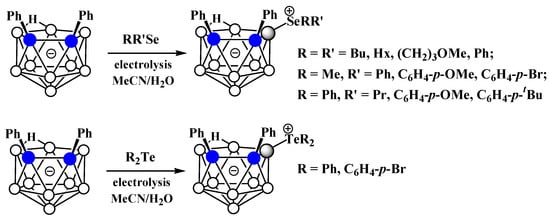
The charge-compensated derivatives of nido-carborane with boron–selenium and boron–tellurium bonds are rather rare. Similar to the dialkyl- and diarylsulfonium derivatives, a series of asymmetrically substituted triakyl(aryl)selenium and triaryltellurium derivatives 9-RR’X-7,8-Ph2-7,8-C2B9H9 (X = Se, Te) were prepared via electrocatalyzed oxidative couplings of 7,8-diphenyl-nido-carborane with RR’Se and R2Te, respectively (Scheme 74, Figure 52) [106].

Scheme 74. Synthesis of 9-dialkyl(aryl)selenium and 9-diaryltellurium derivatives of nido-carborane 9-RR’X-7,8-Ph2-7,8-C2B9H9.

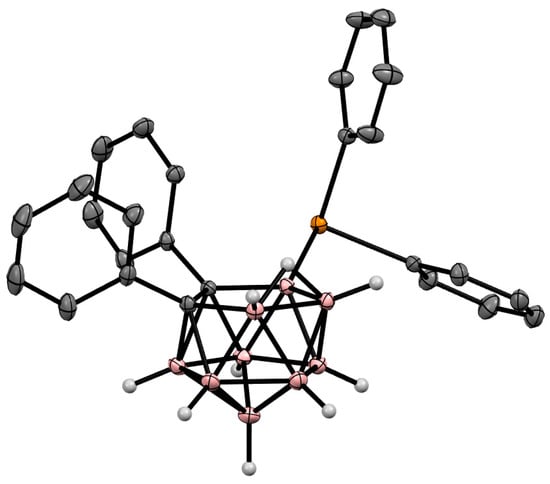
Figure 52. Crystal molecular structure of 9-Ph2Te-7,8-Ph2-7,8-C2B9H9. Hydrogen atoms of phenyl groups are omitted for clarity.
8. Some Other Charge-Compensated Derivatives of Nido-Carborane
The asymmetrically substituted 9-carbonyl derivative of nido-carborane 9-O≡C-7,8-C2B9H11 and the 3,3,8-(CO)3-3,1,2-CoC2B9H10 cobaltacarborane based on its symmetrically substituted analog as a ligand were isolated as minor products of the reaction of the parent nido-carborane with [Co2(CO)8] [161].
The symmetrically substituted cobaltacenium derivative of nido-carborane 10-{CpCo(C5H4)}-7,8-Me2-7,8-C2B9H9 (Figure 53) was prepared along with the 3-Cp-1,2-Me2-3,1,2-CoC2B9H9 cobaltacarborane in the reaction of the dithallium dicarbollide salt Tl2[7,8-Me2-7,8-C2B9H9] with CpCo(CO)I2 in acetonitrile [162].

Figure 53. Crystal molecular structure of 10-{CpCo(C5H4)}-7,8-Me2-7,8-C2B9H9. Hydrogen atoms of methyl groups are omitted for clarity.
This entry is adapted from the peer-reviewed paper 10.3390/inorganics11020072
This entry is offline, you can click here to edit this entry!
