Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Green synthesized cerium oxide nanoparticles (GS-CeO2 NPs) have a unique size, shape, and biofunctional properties and are decorated with potential biocompatible agents to perform various therapeutic actions, such as antimicrobial, anticancer, antidiabetic, and antioxidant effects and drug delivery, by acquiring various mechanistic approaches at the molecular level.
- cerium metal
- cancer
- DNA fragmentation
1. Introduction
Cerium is an iron-gray lustrous element that belongs to the lanthanide group, with an atomic number of 58 and an atomic weight of 140.166 [1]. Cerium was discovered by the Italian astronomer Giuseppe Piazzi in 1801, and he gave it the name ceres. It is an interesting element due to its electronic structure, which displays two variable oxidation states, Ce+3 and Ce+4, due to its constantly changing valance electron positions between (d) and (f) orbitals [2]. Naturally, cerium is a solid ductile metal at room temperature. Cerium is an abundant rare-earth element that makes up to 0.0046% of the earth’s crust [3]. Cerium is malleable (hydroscopic) and can be rapidly oxidized at room temperature, especially in the case of high humidity. Cerium is the most reactive earth metal except for europium, which also belongs to the lanthanide category. Cerium decomposes quickly in hot water and sluggishly in cold water [3]. Cerium and its compounds have many applications in different industries. Cerium oxide is used in the walls of self-cleaning ovens, in incandescent lantern mantles, for polishing glass surfaces, and as a decolorizer for glass [4]. Cerium is a good heat and electricity conductor, along with a potential ultraviolet ray absorber [5]. Due to its special electronic structure, cerium varies between cerium dioxide (CeO2) and di-cerium trioxide (Ce2O3). However, Ce2O3 is an unstable form and is radially converted into CeO2 [6].
Nanotechnology is an emerging field of science, and metallic salt was successfully converted into nanoparticles by American Physicist Dr. Richard Feynman, an early pioneer of nanotechnology. An overview of the use of CeO2 NPs in the biomedical field and the proposed mechanisms is illustrated in Figure 1. There are numerous methods, tools, and techniques available to synthesize CeO2 NPs. Due to their small size and high surface-to-volume ratio, NPs have many applications in different industries, such as pharmaceutical, medical, cosmetic, agricultural, and engineering sectors [7]. CeO2 NPs exhibit unique physical and chemical properties that deviate significantly from the bulk metallic salt, and the positive charge on the CeO2 NP surface allows it to bind various functional groups [8], which are special because of their biological properties. Cerium is a good oxidizing agent, a substance that gains electrons from others by reducing itself in a chemical reaction. Because of this, researchers found that CeO2 NPs can be an important ingredient in the latest medicines that are used against oxidative stress and to treat ailments that arise due to oxidative damage in the human body. Recently, different methods have been implemented to synthesize CeO2 NPs, including solution precipitation, spray pyrolysis, sonochemical methods, solvothermal methods, ball milling, thermal decomposition, sol–gel methods, and thermal hydrolysis (Figure 2) [9]. Basically, there are three categories for the synthesis of CeO2 NPs: chemical, physical, and biogenic. The chemical and physical methods are both expensive. They require hazardous chemicals, which are not eco-friendly and harmful to the living body, and high temperatures and pressures. The living body vigorously reacts to these chemicals, which can generate major health issues rather than solutions [10]. The GS of CeO2 NPs because plants are factories of useful and essential secondary metabolites. There are 452 plant families, and each plant is a reservoir of primary and secondary metabolites. Plants growing in a xeric environment or at high altitudes always store unique types of phytochemicals. The plant extract is a cocktail of phytochemicals that reduce metallic salt to nanosized particles. [4]. Various organic and inorganic polymers bind through ionic and covalent bonds to NP surfaces. The plant extract is a cocktail of numerous organic polymers [11]. These polymers encapsulate NP surfaces during synthesis and decorate them with various functional groups. The biopolymer coating makes CeO2 NPs biocompatible and less toxic entities [12]. Organic coatings stabilize NPs and protect them from nonspecific interactions with cell receptors, proteins, enzymes, and membrane polysaccharides [13]. Plants are small factories of chemicals, and each factory prepares special products that are different from others, such as flavonoids, terpenoids, saponins, glutathione, hydrogen peroxide, ascorbic acid, tannins, caffeine, amines, and nicotine. The coating of these organic compounds on CeO2 NP surfaces makes them safe and efficient for medicinal purposes [14].
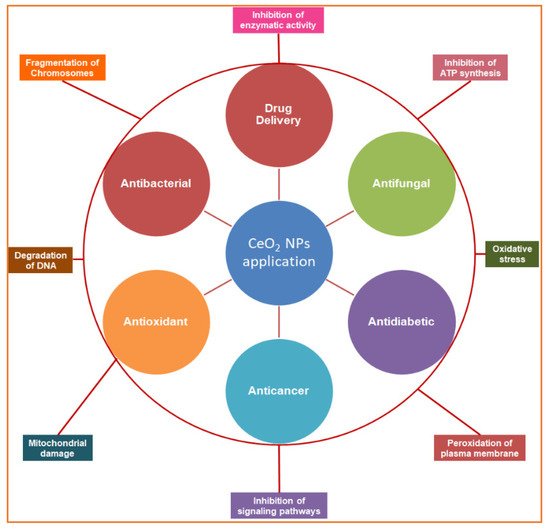
Figure 1. Schematic overview of the study representing the biomedical applications of biogenic cerium oxide nanoparticles (inner circle) and their mechanisms of action (outer circle).
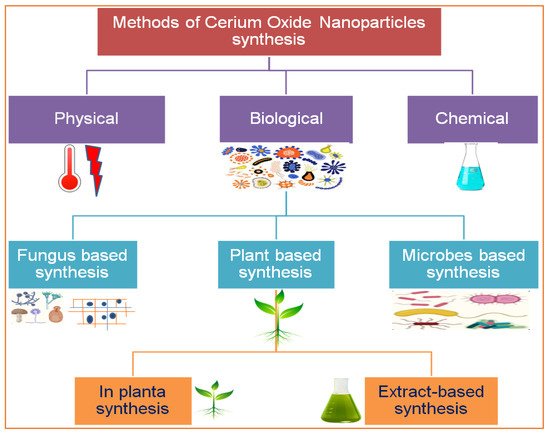
Figure 2. Various routes for the synthesis of cerium nanoparticles.
2. Phytosynthesis of CeO2 NPs and Other Alternative Approaches
There are several methods used to synthesize NPs. Each synthesis technique follows particular protocols to obtain the specific size and shape of NPs [15]. Some methods require special equipment and temperature and pressure conditions to synthesize NPs. Nanomaterials have been prepared through two standard methods for a long time: physical and chemical (Figure 2) [16]. These two methods are not only health-hazardous but also make it costly to obtain a defined amount of nanomaterial [17]. Recently, researchers developed another method termed ‘green syntheses’ to make nanoparticles (Figure 3). The use of plants to synthesize NPs is beneficial in many aspects; for example, they are cost-effective, non-toxic, easy, safe, and time-saving [18]. The efficacy of medicinal plants for the synthesis of nanoparticles is due to their phytochemical contents, which are involved in the synthesis of various shapes and sizes of nanoparticles. Various phytochemicals present in plants, including flavonoids, polyphenols, alkaloids, terpenoids, and low/high-molecular-weight proteins, are involved in the green synthesis of metallic nanoparticles upon the reduction of their precursor salts into nanoparticles and their stabilization in a redox-mediated process (Figure 3). Each plant stores special phytochemicals. Different plant species contain different phytochemical profiles. Most plants synthesize these chemicals for protection against biotic and abiotic stresses [10]. These plants’ phytochemicals can be ketones, carboxylic acids, phenols, ascorbic acids, amines, carbonyl, hydroxyl, and benzene [19]. The different parts of plants that are used to prepare herbal extracts include roots, stems, leaves, bark, flowers, pollen, or the whole plant body. Many studies are available that have utilized plant-derived biological products, such as resins, gums, nectar, chitosan, and juice, as reducing agents [20]. The use of fresh plant material for extract preparation is superior and health-conscious because the plant body contains many volatile chemicals, hormones, enzymes, vitamins, proteins, and micro and macro trace elements that are not present in plant-derived biological products [21]. During green synthesis, the functional groups present in a plant extract encapsulate NP surfaces and convert them into less toxic, biocompatible, and biodegradable products. One study reported that GS CeO2 NPs rarely show drug toxicity and are drug-resistant because of their organic nature [22]. The green synthesis of nanoparticles is considered a good replacement to overcome the challenges of cytotoxicity and genotoxicity [23]. Another study explained that GS CeO2 NPs did not produce any allergic reactions in the case of a surgical wound. Three steps are involved in GS CeO2 NPs: (i) nucleation/fabrication, (ii) growth/development, and (iii) encapsulation/coating. The first step is the nucleation of metallic salt into same-sized particles; second is the growth and development of nuclei (all nuclei are not formed at once because this causes the agglomeration of NPs), and in the third step, NPs are emulsified and decorated with plant functional groups [24].
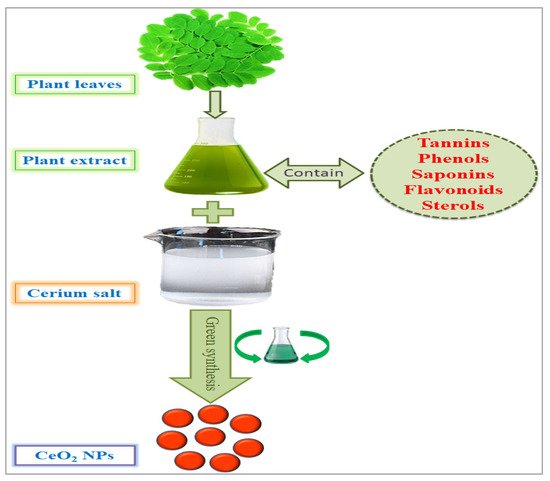
Figure 3. Green synthesis of cerium nanoparticles and encapsulation with plant phytochemicals.
In previous research, researchers have used various plants to prepare GS CeO2 NPs of different sizes and shapes (Table 1). Prosopis juliflora (Sw.) DC. leaf extract-mediated spherical CeO2 NPs 15 nm in size were fast enough to cross the cell membrane [25]. Similarly, in another study, Punica granatum L. fruit extract was used to fabricate 10 nm spherical CeO2 NPs that were small enough to reach the genetic material of cells by crossing the nuclear membrane. Sida acuta. whole plant extract-mediated CeO2 NPs 8.2 nm in size were found to be a feasible carrier to the blood–brain barrier to deliver drugs to brain cells [25]. The leaf extract of Justicia adhatoda L. and Origanum majorana L.-mediated CeO2 NPs with a 200 nm diameter were observed to be good entities for delivering drugs through the bloodstream [26]. One study demonstrated the use of tannic acid and pectin to synthesize spherical CeO2 NPs with 20 nm and 40 nm diameters, which are small enough to deliver drugs inside the cell [27]. Nanomaterials are characterized by different techniques: scanning electron microscopy (SEM) to determine the size of NPs, transmission electron microscopy (TEM) to provide information about shape and surface morphology, UV–visible techniques to measure NP concentrations in a solution, Fourier transform infrared spectroscopy (FTIR) to inform about chemical bonds on the NP surface [28], and energy-dispersive X-ray spectroscopy (EDX) to confirm the chemical nature of the compounds used to synthesize nanomaterials [29].
Table 1. Cerium oxide nanoparticle synthesis by using various routes and their biomedical applications.
| No. | Plant Name | Plant Part Used | Size of NPs | Activity | References |
|---|---|---|---|---|---|
| 1 | Calotropis procera | Flower | 20 nm | Biogenic CeO2 NPs exhibited important antibacterial activity against E. coli and Pseudomonas. | [30] |
| 2 | Solanum nigrum L. | Leaves | 20 nm | Biosynthesized CeO2 NPs exhibited the highest antibacterial activity against Gram-positive Bacillus subtilis and Gram-negative against E. coli. | [31] |
| 3 | Aloe barbadensis | Gel | 10 nm | Green CeO2 NPs showed high antioxidant potential. | [32] |
| 4 | Olea europaea L. | Leaves extract | 24 nm | Successful inhibition of fungal and bacterial strains. |
[33] |
| 5 | Azadirachta indica | Leaves | 50 nm | CeO2 NPs exhibited a good photo-degradation rate. |
[34] |
| 6 | Gloriosa superba L. | Leaves | 5 nm | CeO2 NPs exhibited good photoluminescence and antibacterial activities against Gram-positive and Gram-negative species. |
[35] |
| 7 | Citrullus lanatus | Juice | 11.6 nm | Biosynthesized CeO2 NPs exhibited good photocatalytic activity and antibacterial potential by causing leakage of the bacterial membrane. | [36] |
| 8 | Prosopis fracta | Fruit | 15 nm | Green synthesized CeO2 NPs showed cellular toxicity against colon cancer cells. |
[37] |
| 9 | Prosopis fracta | Aerial parts (leaves, branches) |
30 nm | Biosynthesized CeO2 NPs were found to be less effective against HT-29 cancer cells. | [38] |
| 10 | Camellia sinensis L. | Leaves | 5 nm | Biogenic CeO2 NPs were found to be protective against the oxidation of hepatic inflammation and oxidation of hepatic cells. |
[38] |
| 11 | Humicola sp. | Fungus mycelia | 5 nm | Biosynthesized CeO2 NPs were found to be highly stable and did not agglomerate in an aqueous solution. | [39] |
| 12 | Salvadora persica L. | Whole plant extract | 20 nm | Green synthesized CeO2 NPs were found to be effective against a breast cancer cell line (MCF-7). | [119] |
| 13 | Musa sapientum L. | Fruit | 13 nm | Biosynthesized CeO2 NPs were found to be good sun-protective agents and anticancer agents against a lung (A549) cancer cell line. |
[40] |
| 14 | Acalypha indica L. | Leaves | 15–30 nm | Biogenic CeO2 NPs showed antibacterial behavior against Gram-positive and Gram-negative species. | [41] |
| 15 | Brassica napus L. | Pollen grains | 4 nm | Green CeO2 NPs destroyed ovarian cancer cells (A2780). |
[42] |
| 16 | Aspergillus niger | Fungus mycelia | 5–20 nm | Green CeO2 NPs exhibited high insecticidal potential against Aedes aegypti and antibacterial activity against Streptococcus pneumonia, Bacillus subtilis. |
[43] |
| 17 | Origanum majorana L. | Leaves | 70 nm | Green synthesized CeO2 NPs could express SOD, CAT, POX, and antioxidant activities and were found to be highly cytotoxic against the MDA-MB-231 cancer cell line. |
[28] |
| 18 | Prosopis juliflora | Leaves | 3.7 nm | Green synthesized CeO2 NPs were highly effective in killing both Gram-positive bacteria (Staphylococcus aureus, Streptococcus pneumonia) and Gram-negative bacteria (Pseudomonas aeruginosa, Proteus vulgaris). |
[44] |
| 19 | Aloe vera (L.) | Leaves | 2–3 nm | Biogenic CeO2 NPs were found to be highly antioxidant agents. | [45] |
| 20 | Petroselinum crispum | Fruit | 25 nm | Green CeO2 NPs exhibited high antioxidative activity against various stresses in agricultural plants. |
[46] |
| 21 | Musa sapientum L. | Peel extract | 4–13 nm | Green CeO2 NPs exhibited high photocatalytic activity. |
[47] |
| 22 | Acorus calamus L. | Rhizome extract | 22.03 nm | Biogenic CeO2 NPs showed good antibacterial activity against Gram-positive and Gram-negative species. |
[48] |
| 23 | Moringa oleifera | Seed | 30 nm | Green CeO2 NPs were found to express suitable insecticidal activity. | [49] |
| 24 | Hibiscus Sabdariffa L. | Flower | 3.9 nm | Green synthesized CeO2 NPs were found to be highly effective chelating agents. | [50] |
| 25 | Amomum subulatum | Seeds | 0.5 µm | Green CeO2 NPs were found to be highly effective against MRSA, methicillin-resistant S. aureus infection, which primarily affects animal mammary glands. |
[51] |
| 26 | Aloe vera (L.) | Leaves | 7–12 nm | Green CeO2 NPs showed good optical properties at different concentrations of nanoparticles. | [52] |
| 27 | Sida acuta | Leaves | 8.2 nm | Green synthesized CeO2 NPs disrupted the cell membrane of E. coli and killed bacteria. |
[26] |
| 28 | Rheum turkestanicum | Whole plant | 30 nm | Green synthesized CeO2 NPs exhibited photocatalytic and cytotoxic activities against PC12 cell lines. | [53] |
| 29 | Saccostrea cucullata | Whole mollusk | 15 nm | Biogenic CeO2 NPs exhibited suitable photocatalytic and cytotoxic activities. |
[54] |
| 30 | Ceratoniasilique L. | Leaves | 100 nm | Green synthesized CeO2 NPs were found to be effective against the hepatic cancer cell line. | [3] |
3. Physicochemical Parameters Affecting the Synthesis of Green Cerium Oxide Nanoparticles
Distinct physicochemical reaction parameters, for instance, cerium salt, pH, temperature, and the proportion of biological extract, work collectively to control the molecular dynamics, reaction kinetics, enzymatic reactions, and protein conformations that affect the size, shape, and biochemical properties of nanoparticles [55,56]. Different physicochemical reaction conditions determine the different morphologies of nanoparticles, such as polygonal, cubic, spherical, round, crystalline, and octahedral. Physicochemical parameters perform the function of a toolkit to sculpt and trim the nanoparticles into various sizes and shapes [57]. Biogenic synthesis of nanoparticles is a safe, less toxic technique that has recently been utilized by researchers. This technique uses different biological resources, including plants, microbes, algae, fungi, or any other biologically derived products [58]. These biological extracts are a rich source of biochemicals, such as terpenoids, saponins, flavonoids, amines, ketones, phenols, carboxylic acid, glutathione, hormones, minerals, vitamins, and enzymes that are involved in the reduction of metallic salt into nanomaterial [59]. Plants are considered the agents with the most potential due to their abundance, safe and unharmful nature, and the fact that they are the large factories of phytochemicals/secondary metabolites that are free of chemical danger. Different plant parts, such as leaves, stems, roots, flowers, fruit, pollen, bark, and wood, store phytochemicals of variable nature according to their role in the plant life cycle [58]. For that reason, each plant part contains a variable proportion of phytochemicals that determine the amount of plant material to utilize for the green synthesis of nanomaterials. Secondary metabolites are differentiated into organic and inorganic chemicals, including oil, gum, resins, hormones, nectar, and ascorbic acid (6). This is why the plant body is considered the bank of several functional groups that coat and charge nanoparticles’ surfaces in green synthesis [60]. However standard physicochemical parameter measurements for the green synthesis of nanoparticles vary from plant to plant. Physicochemical reaction conditions participate directly to control the size, shape, and yield of cerium oxide nanoparticles [56]. Temperature is actually the source of energetic electrons, and their flow also energizes other sources that they strike. Temperature provides kinetic energy that accelerates the chemical reaction. Temperature kinetic energy acts as the activation energy that is usually required to start the chemical reaction. Temperature triggers molecular collision that ensures coalescence between the phytochemical extract and cerium salt and converts the solution into the final product [61]. The surface charge of nanoparticles is also controlled by the pH of the solution. The pH value constantly varies during nanoparticle synthesis. Variable pH conditions have a different impact on the reaction kinetics and molecular mechanisms. The increase or decrease in pH value determines the number of H+ ions in the reaction solution. A higher pH value is responsible for low H+ ions, and lower pH results in more H+ available in the reaction mixture. The pH does not contribute to the determination of nanoparticles’ shape and size, but changes in pH influence the electronegative properties and oxidation states by inhibiting the enzyme’s active site, reducing its binding ability, which decreases the rate of the reaction and finally yields nanoparticles [62]. The proportion of reactants directly influences the catalytic property of the reaction mixture as well as the quantity of metallic salt, which both collide in a synergistic way to enhance the nanoparticle yield. The proper mass of the metallic salt and the appropriate volume of the plant extract ensure the presence of an equitable number of reducing and oxidizing agents in the reaction mixture [63]. The selection of physicochemical reaction parameters influences the morphological, physiochemical, and charge-carrying properties of cerium oxide nanoparticles, which affect their biocompatibility, bioaccessibility, biodegradability, and reactivity for treating different diseases [64].
This entry is adapted from the peer-reviewed paper 10.3390/nano12122117
This entry is offline, you can click here to edit this entry!
