Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The epithelial to mesenchymal transition (EMT) is a process by which cells exhibiting an epithelial phenotype adopt a mesenchymal phenotype, which facilitates migration, invasion, and metastasis. This entry provides an overview of the role, regulation, and targeting of EMT in Osteosarcoma, a primary bone malignancy affecting mainly children and young adults.
- osteosarcoma
- epithelial-mesenchymal transition
- transcriptional regulation
1. Introduction
Osteosarcoma (OS) is a primary bone malignancy with an annual incidence of 2–4 per million [1]. It typically affects children, teens, and young adults [2], with a peak incidence from ages 10–19 [1], a second peak in adults over 60 [2], and a slight male preponderance [3]. The overall 5-year survival rate for OS is 60% but decreases to 27% in the presence of distant metastases [4]; the rate of metastases at diagnosis is 18% [5].
The origin of OS is poorly understood. As a sarcoma, it arises from mesenchymal cells, but it is not currently known whether the precursor cells are osteoblasts or mesenchymal stem cells [6]. Although the etiology of OS is largely a mystery, multiple risk factors have been identified. These include medical conditions such as hereditary retinoblastoma, Li-Fraumeni syndrome, Werner syndrome, Rothmund-Thompson syndrome, Bloom syndrome, and Paget’s disease [3]. Other risk factors include exposure to ionizing radiation and alkylating agents, both of which may have been used in the treatment of a prior malignancy [3].
The mainstay of treatment for osteosarcoma is surgical resection and frequently involves both neoadjuvant and adjuvant chemotherapy for higher grade tumors [7]. While advances in surgical techniques and chemotherapeutic regimens were associated with an initial improvement in outcomes, overall survival in OS has not significantly changed in several decades [8]. As medicine becomes more personalized, there is a growing interest in the identification of novel targeted therapies. A key component in developing targeted therapy is identifying specific pathways, proteins, or other molecules essential to cancer cell function. One of the cellular features often associated with aggressive cancers is the epithelial to mesenchymal transition (EMT).
2. EMT in Cancer
EMT is depicted in Figure 1. It is a process by which cells exhibiting an epithelial phenotype adopt a mesenchymal phenotype, which facilitates migration, invasion, and metastasis [9]. It exists in equilibrium with a reverse and complementary process, the mesenchymal to epithelial transition (MET), wherein cells revert back to an epithelial phenotype. Primary epithelial tumors exhibit epithelial cell markers such as E-cadherin. These cells demonstrate apical polarity, adhesion to a basement membrane, and tight cellular junctions [10]. For many cancers, EMT is critical in the early transition from normal to malignant cells. It is characterized by downregulation of epithelial cell markers, destabilization and loss of cell–cell junctions, loss of adherence to basement membrane and apical polarity, and cytoskeletal reorganization [9]. The result of these changes is a cell with mesenchymal morphology and characteristics.
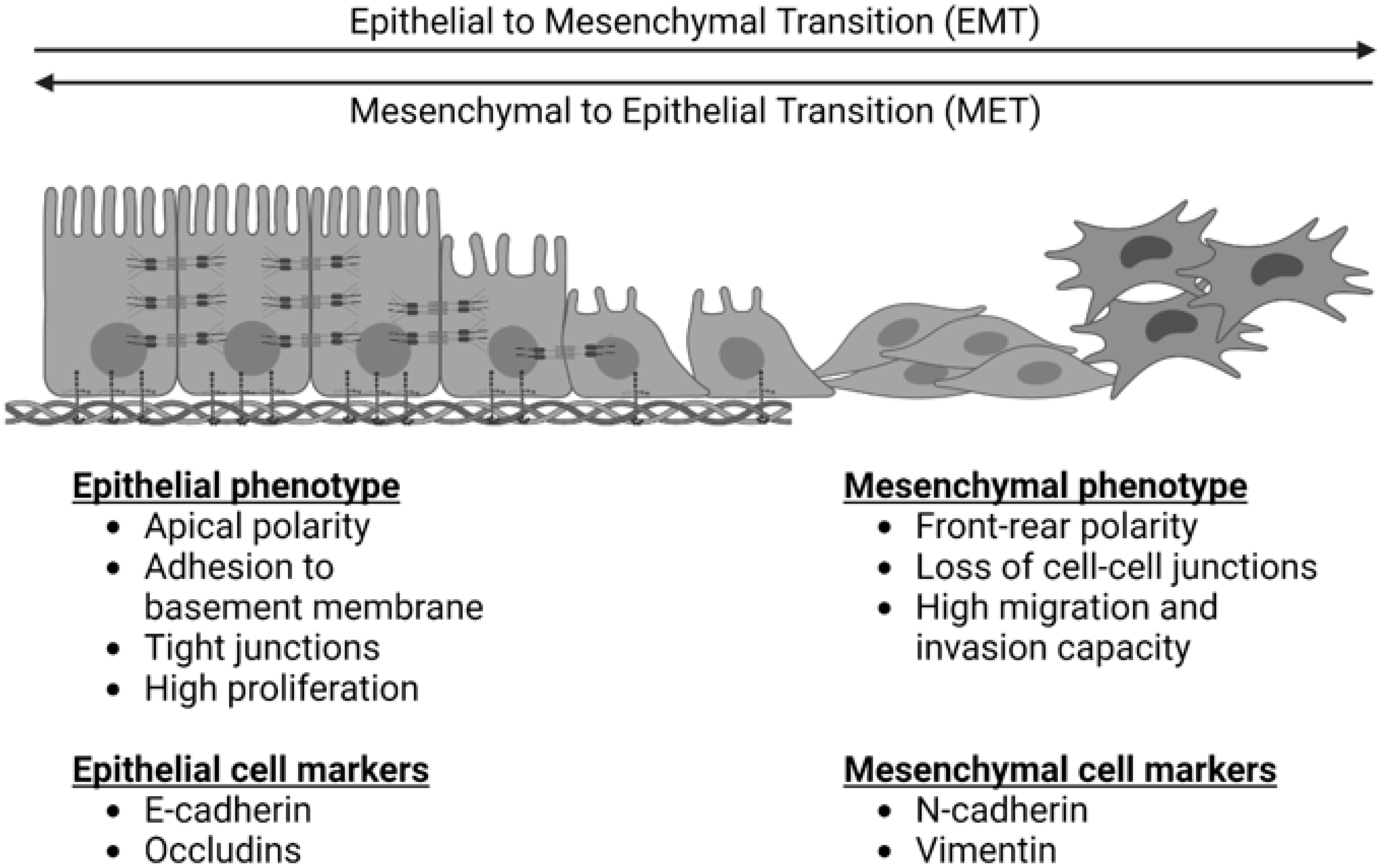
Figure 1. The epithelial to mesenchymal transition (EMT) and the reverse process of the mesenchymal to epithelial transition (MET). EMT is characterized by a loss of epithelial cell markers, an increase in mesenchymal cell markers, a loss of apical cell polarity, a loss of tight cell junctions, and an increased capacity for cell migration and invasion. MET is characterized by a loss of mesenchymal cell markers, an increase in epithelial cell markers, increased apical cell polarity, tight junctions, adherence to a basement membrane, and increased cell proliferation.
Given the migratory potential of mesenchymal cells compared to epithelial cells, EMT has long been linked to cancer metastasis. However, inhibition of EMT has not been shown to affect the establishment of cancer metastases in vivo [11][12], and the cells found within metastatic tumors are more likely to exhibit an epithelial phenotype [12][13]. Despite this, tumor cells that have undergone EMT appear to drive local invasion and angiogenesis of the primary tumor [13]. These results suggest that EMT is critical for tumor invasion into the local vascular system, allowing cells to migrate to distant organs where secondary tumors are established largely by cells with an epithelial phenotype, which have a greater propensity for proliferation [9]. These may be either cells that have undergone EMT and subsequently MET or primary tumor cells that did not undergo EMT [13].
The molecular pathways associated with EMT are summarized in Figure 2. Zinc-finger E-box binding homeobox (ZEB), snail family transcriptional repressor 1 (SNAIL), snail family transcriptional repressor 2 (SLUG), and twist-related protein (TWIST) are well-known EMT transcription factors that are established downstream targets of multiple signaling pathways, including the canonical wnt/β-catenin pathway, the neurogenic locus notch homolog protein (Notch) pathway, the Transforming Growth Factor β/Suppressor of Mothers Against Decapentaplegic (TGFβ/SMAD) pathway, the phosphoinositide 3-kinase (PI3K)/Akt pathway, the mitogen-activated protein kinase (MAPK) pathway, the Ras/Raf/Mitogen-activated protein kinase/ERK kinase/extracellular-signal-regulated kinase (RAS/RAF/MEK/ERK) axis, and the Janus kinase-Signal Transducer and Activator of Transcription JAK/STAT pathway [10]. These signaling cascades often interact, share many intermediaries, and impact the regulation of one another. This presents a challenge for studying and targeting EMT, as the individual pathways are difficult to isolate.
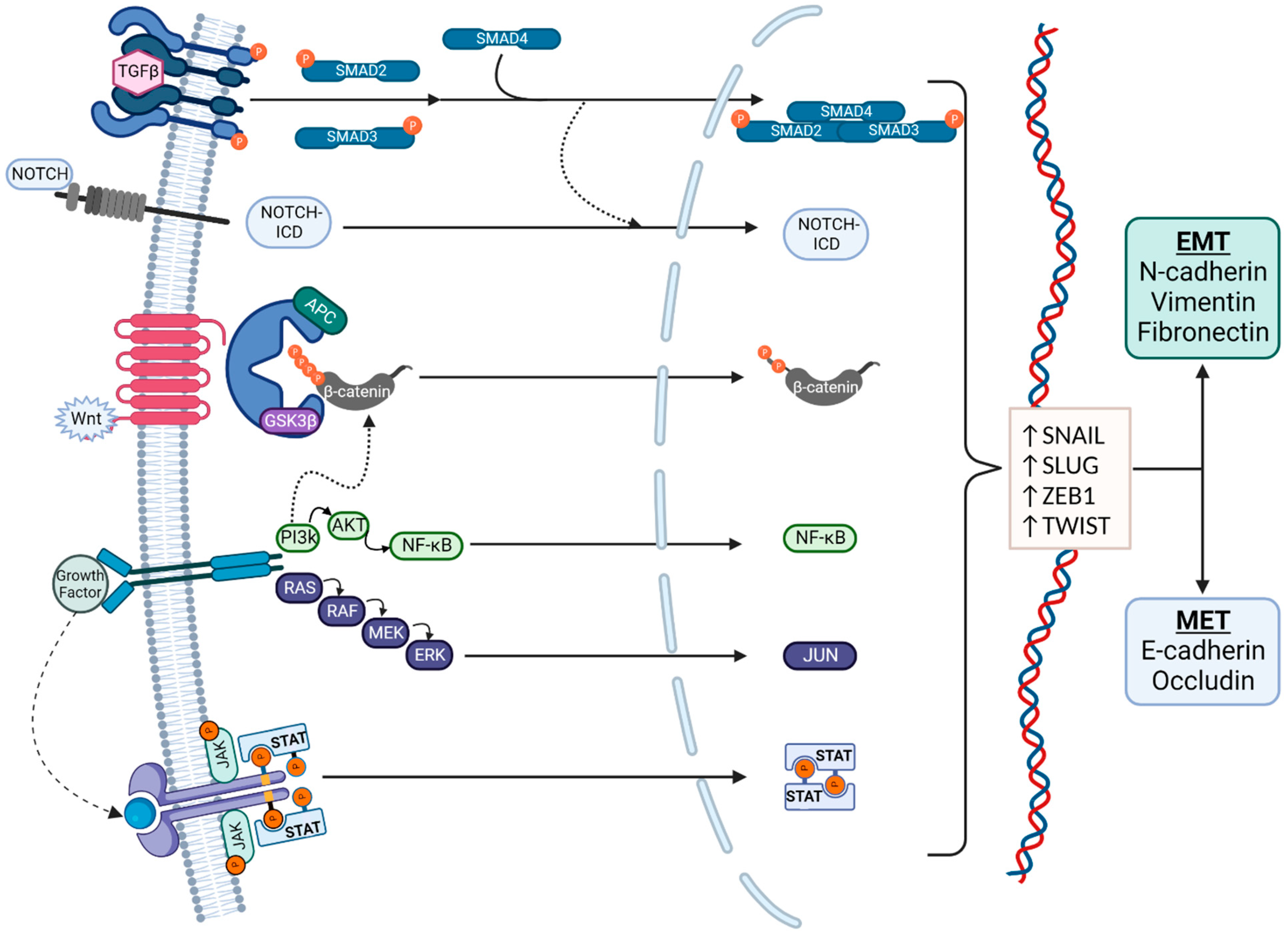
Figure 2. Signaling pathways in EMT. EMT regulation is complex and affected by multiple pathways, which also interact with each other. Regulation is typically via the Transforming Growth Factor β (TGFβ)/SMAD, Notch, canonical wnt, phosphoinositide 3-kinase (PI3K)/Akt, RAS/RAF, and JAK/STAT pathways. The transcription factors that mediate EMT are primarily Snail, Slug, ZEB1, and TWIST. EMT is characterized by an increased production of N-cadherin, vimentin, and fibronectin, and MET is characterized by an increased production of E-cadherin and Occludin.
3. EMT Signaling Pathways
3.1. TGFβ/SMAD Pathway
The TGFβ family of proteins includes three TGFβ isoforms, activins, and bone morphogenic proteins (BMPs) [14]. In EMT, TGFβs bind to TGFβ receptors (1/2), which initiate a signaling cascade, leading to the increased transcription of genes involved in EMT. Binding of TGFβ to its receptors (1/2) leads to phosphorylation of SMAD2 and SMAD3, which then form a complex with SMAD4. BMPs also bind TGFβ receptors, activating SMAD1 and SMAD5 and then forming a complex with SMAD4. These trimeric complexes migrate to the nucleus to act as transcription factors.
SMAD complexes activate the mesenchymal genes vimentin and fibronectin, as well as the EMT transcription factors Snail, Slug, Zinc finger E-box-binding homeobox 1 (ZEB1) and Twist. These, in turn, repress E-cadherin and can upregulate the expression of TGFβ ligands, establishing a positive feedback loop to maintain EMT [9][15][16].
3.2. Canonical wnt Pathway
The canonical wnt pathway is considered to be a key activator of EMT [9]. Signaling is initiated by a group of wnt ligands that bind to Frizzled receptors and trigger a cascade of events, leading to the nuclear translocation of β-catenin. β-catenin is constitutively produced in the cell and stored in cytosolic pools. In the absence of wnt signaling, phosphorylated β-catenin is associated with a destruction complex, ubiquitinated, and degraded by proteasomes. Following activation of the canonical wnt pathway, β-catenin is dephosphorylated and translocates to the nucleus, where it acts as a transcriptional co-factor to induce the expression of genes involved in cell differentiation, proliferation, and tumorigenesis [17][18].
This pathway has been directly implicated in EMT via the expression of Twist, Slug, N-cadherin, and the repression of E-cadherin [19]. The known EMT transcription factor Snail has been shown to positively regulate wnt signaling [20]. The inhibition of Secreted Frizzled Related Protein 1 (SFRP), a negative regulator of wnt ligands, has also been shown to have EMT-like effects in breast carcinoma cells in vitro, while sensitizing them to TGFβ-induced EMT [21].
β-catenin is also located at the cell membrane as part of an E-cadherin-containing multi-component adherens junction complex, which is a component of cell–cell interaction junctions. β-catenin contributes to anchoring E-cadherin, a transmembrane cell–cell adhesion protein at the cell surface to the intracellular actin cytoskeleton. β-catenin is released from the adherens complex upon disruption of these adherens junctions between cells. Once available in the cytosol, it enters the pathway described above and is either phosphorylated and degraded or, if the wnt pathway is active, dephosphorylated and translocated to the nucleus to function as a transcription factor for EMT-genes [22]. E-cadherin can therefore act as a negative regulator of the canonical wnt pathway by sequestering most of the β-catenin in the epithelial cell membrane.
3.3. Notch Pathway
The Notch pathway has been implicated in inducing EMT in both normal and neoplastic tissues, and is involved in controlling cell fate, differentiation, and proliferation. Four isoforms (Notch1 through Notch4) are known to bind Delta-like or Jagged family ligands. This interaction triggers a series of proteolytic events leading to the active fragment Notch Intracellular Domain (Notch ICD), which then acts in the nucleus, where it associates with binding partners and transcriptional activators [23]. Several components of the Notch pathway are highly expressed at the invasive margins of tumors, which express EMT markers such as vimentin, suggesting an important role for the Notch pathway in the regulation of EMT [24]. Notch acts via transcriptional regulation of ZEB, Snail, and Slug, which repress expression of E-cadherin and induce expression of vimentin and fibronectin [23][24][25].
There is crosstalk between the Notch and TGFβ pathway that occurs via SMADs. As described above, the SMAD family of proteins are integral to TGFβ signaling. They have also been shown to associate with Notch-ICD. This affects the expression of genes downstream of both Notch and TGFβ that are required for mesenchymal differentiation, a key component in EMT [26]. Silencing components of the Notch pathway have also been shown to prevent TGFβ-induced EMT [27].
3.4. Tyrosine Kinase Pathways
Mitogenic growth factors also play a role in the regulation of EMT. The binding of these growth factors causes their receptors to dimerize and induces the activation of both receptor and non-receptor tyrosine kinases (TKs). This enables the activation of several pathways—including the MAPK, JAK-STAT, and phosphatidylinositol 3-kinase-Akt (PI3-Akt) pathways. All of these have been implicated in EMT, and are involved in cell growth, proliferation, and migration [28]. PI3K/Akt has also been shown to play an important role in the regulation of transcriptionally active β-catenin, a key molecule in the previously discussed wnt signaling pathway [29]. Inhibition of TKs is a growing field of study in cancer therapeutics, with multiple inhibitors currently under investigation [30].
Inhibition of fibroblast growth factor (FGF), a mitogenic growth factor that participates in the induction of EMT via activation of the MAPK, induces the reverse process MET in vitro and delays tumor growth in vivo [31]. One isoform, FGF2, has been associated with reduced overall survival in several carcinoma types if overexpressed [31].
The binding of epidermal growth factor (EGF) to its receptor leads to activation of MAPK pathway and decreased expression of E-cadherin [32]. EGF also activates the JAK2-STAT3 pathway, which leads to EMT activation via Twist [33]. Additionally, EGF has been shown to induce EMT via TGFβ signaling and regulation of Snail [34] and phosphorylation of SMAD2/SMAD3 [35].
The activation of Akt, or Protein Kinase B, has been shown to upregulate the phosphorylation of Twist1 and inhibit apoptosis [36], and the inhibition of Akt has been shown to induce MET [37]. For example, hepatocyte growth factor (HGF) has been shown to activate EMT [38], which can be reversed via inhibition of the PI3K/Akt pathway. HGF was found to enhance tumor progression and metastasis of hepatocellular carcinoma in association with the c-MET receptor tyrosine kinase [39], a known activator of PI3K/Akt.
4. EMT in OS
As a mesenchymal cancer, the importance of EMT in OS has been disputed [40][41]. In fact, an early investigation including 4 clinical osteosarcoma samples by Sato et al. found there was no detectable E-cadherin expression in these cells [42], suggesting that the repression/downregulation of E-cadherin—a classically described step in EMT—would not be possible. In contrast, Yin et al. found that 20.6% of OS tissue samples expressed E-cadherin and those that did were less likely to metastasize, whereas the expression of Twist was significantly related to metastases and poorer overall survival [43]. The promotion of EMT in OS characterized by increased migration and invasion in vitro has been shown to be mediated via upregulation of Snail [44][45][46][47][48][49][50][51][52][53][54][55][56][57], Slug [58][59], Twist [60][61][62][63], and ZEB [64][65][66][67].
The following sections give an overview of studies that have examined the roles of different EMT regulatory molecules in OS. All of these were found to affect the expression of EMT-related cell-markers and are correlated closely with EMT-associated cellular features such as increased migration and invasion. Many also showed a link between their proposed EMT-regulatory molecule and OS metastases in vivo in animal models. Taken together, these results suggest that EMT does play a role in osteosarcoma and is associated with a more aggressive tumor phenotype. However, the term “transition” is not ideally suited to sarcoma cancers, and EMT may be better thought of as a set of pathways utilized to maintain and promote the existing mesenchymal phenotype.
Sannino et al. posited a possible hybrid phenotype in sarcoma tumors cells, utilizing the EMT and MET pathways to acquire both mesenchymal and epithelial characteristics that favor the initiation and establishment of distant metastases [40].
The highlighted pathways important in EMT regulation have all been shown to have a role in OS. TGFβs promote EMT and metastases in OS [68], and TGFβ inhibition has been shown to decrease EMT in OS [58][69][70][71][72][73][74]. Chen et al. also identified that estrogen-related receptor α (ERRα) upregulates TGF-β-mediated EMT in two OS cell lines [50]. Others have highlighted roles for MAPK [63][75][76][77] and JAK/STAT [52][78][79][80][81][82].
Notch signaling promotes proliferation, migration, and invasion of OS cells, and Notch overexpression increased tumor growth in vivo [83][84][85][86]. Notch inhibition reduced chemo-resistance in OS in vitro [87][88]. Wnt signaling has also been shown to mediate EMT in OS [49][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104]. It has been proposed that wnt signaling is particularly important in the pathogenesis of OS cancer stem cells [105].
TKs are of particular interest in OS, and multiple different TK proteins have been associated with aggressive cellular phenotypes. Many studies have demonstrated a regulatory role in OS for the downstream TK pathways PI3K/Akt [106][107][108][109][110][111][112][113][114] and RAS/RAF/MEK/ERK [61][115][116][117][118]. Multiple TK inhibitors have been a part of recently completed or ongoing clinical trials in the treatment of OS, including Apatinib, Axitinib, Cabozantinib, Cediranib, Crizotinib, Dasatinib, Imatinib, Pazopanib, Regorafenib, Sorafenib, and Sunitinib [119][120].
5. Regulation of EMT in OS—Proteins
As a complex and multi-faceted process, several proteins have been implicated in EMT regulation in OS [44][48][49][53][54][55][61][62][64][78][89][91][93][94][97][100][106][107][108][109][112][113][114][116][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151]. These proteins were found to either promote [44][48][53][54][61][64][78][89][93][94][97][100][106][107][108][109][112][113][114][116][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146] or inhibit [49][55][62][91][147][148][149][150][151] EMT in vitro, and the majority were found to be correspondingly upregulated or downregulated in clinical OS tissue samples and/or established cell lines compared to normal controls. Each group of authors found a significant correlation between the studied protein and the levels of EMT-related proteins, such as E-cadherin, N-cadherin, and vimentin. They also reported a significant effect on aggressive cellular characteristics, such as migration and invasion ability in vitro. Where noted, the results were confirmed in vivo with mouse xenograft experiments.
A detailed review of the individual proteins investigated for their regulatory role in EMT of OS cells is outside of the scope of this review. Generally, their endogenous functions can be grouped into the following families: cell cycle regulation, immunity/inflammatory, cell signaling, cell structure, and metabolism. Each of these categories has a logical impact on EMT and/or cancer cell behavior.
Changes to cell differentiation and cell cycle regulation are recognized mechanisms by which normal cells can become cancerous. We identified 22 proteins with a regulatory role in EMT in OS whose endogenous functions impact these processes [53][54][62][64][78][91][97][100][112][116][126][130][131][139][140][141][142][143][144][145][146][151]. This group can be represented by several ubiquitin ligases [142][143] and deubiquitinases [97][145][146] that are known to target proteins critical for cell growth, proliferation, and differentiation. These were all found to be upregulated in clinical samples of osteosarcoma, and the overexpression or inhibition of these proteins was found to correlate with markers for EMT and OS cell proliferation.
The importance of immunity and inflammation on cancer progression is widely recognized [152], and these systems have also been implicated in the regulation of EMT [153]. Ren et al. found that PD-L2 knockdown decreased EMT and inhibited migration, invasion, and colony formation of OS cells in vitro, and reduced OS metastases in vivo in a mouse model [137].
Many of the described proteins are implicated in cell signaling [55][93][94][108][113][126][129][134][135][139][148]. In addition to cell–cell interactions, this broad grouping includes the regulation of multiple cell processes that affect multiple other pathways and functions, including but not limited to cell cycle regulation, inflammation, immunity, and metabolism.
Finally, a subset of the proteins associated with EMT in OS are either structural proteins or regulate cell structure via interaction with the cytoskeleton [89][106][121][122][129][148]. This is perhaps the simplest and most logical grouping given the key morphological changes that take place during the EMT transformation, as depicted in Figure 1. Interestingly, Yuan et al. found that Erythrocyte Membrane Protein Band 4.1-like 3 (EPB41L3)—a cytoskeletal protein involved in cytoskeletal rearrangement, intracellular transport, and signal transduction—was increased in OS tissues and cell lines but was associated with an inhibition of EMT, migration, invasion, and cell viability in OS cell culture [129]. This pattern of expression was opposite to all of the other proteins impacting EMT in OS identified in this review.
When reported, the EMT pathways most implicated in these studies were wnt and PI3K/Akt. The nuclear localization and, therefore, transcriptional activity of the wnt/β-catenin pathway has also been shown to be regulated by PI3K [30], suggesting overlap in these EMT control mechanisms. The most frequently identified downstream target was Snail, which is known to promote EMT by suppressing E-cadherin expression [154], and further upregulates wnt signaling and EMT [20].
6. Regulation of EMT in OS—Non-Coding Ribonucleic Acids
Another key group of regulatory factors of EMT/MET in OS are non-coding ribonucleic acids (ncRNAs). These molecules have many forms and functions [155], one of which is gene regulation. Typically identified through queries to the Gene Expression Omnibus (GEO), the differential expression of multiple separate long non-coding RNAs (lncRNAs) [156], microRNAs (miRNAs) [157], circular RNAs (circRNAs) [158][159], and pseudogenes [160] have been found to relate to OS prognosis [161]. Again, they were found to have a role in either promoting [57][63][66][76][85][86][90][95][98][99][101][103][109][162][163][164][165][166][167][168][169][170][171][172][173][174][175][176][177][178][179][180][181][182][183][184][185][186][187][188][189][190][191][192][193][194][195] or inhibiting [47][49][51][65][77][92][96][102][104][111][196][197][198][199][200][201][202][203][204][205][206][207][208][209][210][211][212][213][214][215][216][217][218][219][220][221][222][223][224] EMT and invasive cellular behaviors of OS cells in vitro, and there was significant overlap in the affected pathways and ultimate downstream targets. Very frequently there are multiple non-coding RNAs involved in the same pathway as they can also regulate other nucleic acids.
Unlike the pattern observed in the majority of these findings, Yuan et al. found that although erythrocyte membrane protein band 4.1-like 3 (EPB41L3) was upregulated in OS cell lines and clinical tissue samples, knockdown of EPB41L3 significantly increased the migration and invasion capacity of the investigated cell lines despite decreased cell viability [130]. The findings were similarly mixed for lncRNA NKILA [200] and miR-let-7d [224]. These studies highlight the complexity of EMT regulation in OS and suggest that it is only one possible factor relating to tumor behavior and prognosis.
7. Regulation of EMT in OS—The Tumor Microenvironment
There has been increased recognition of the importance of the tumor microenvironment on various cellular functions and characteristics. This is the three-dimensional structure surrounding tumor cells and comprises immune cells, vascular network, and extra-cellular matrix (ECM), among other components. The tumor microenvironment is unique not only for different cancer types but also for individual patients, and it is influenced by multiple factors, including patient sex and presence of metastases [225]. A better understanding of the interactions within the tumor micro-environment is expected to lead to the development of personalized treatments targeted at individual patients’ tumors.
Han et al. found that the presence of tumor-associated macrophages (TAMS) and the expression of the inflammatory marker cyclo-oxygenase 2 (COX2) correlated with OS metastases in clinical samples [80]. They also found co-culture of OS cells with TAMS promoted EMT and aggressive cellular features in vitro, which was reversible by COX2 inhibition. Additionally, COX2 inhibition reduced pulmonary metastases in vivo in a murine model [80]. Ling et al. found that Von Willebrand Factor (VWF)—which is secreted by the endothelial cells lining blood vessels—promoted EMT in vitro following OS and endothelial cell co-culture, as well as tumor growth and metastasis in vivo in a mouse model [226].
In addition to the cellular and biochemical makeup of the tumor microenvironment, the biomechanical properties of the ECM may also play a role in regulating EMT. Dai et al. developed a three-dimensional cell culture model with varying degrees of ECM stiffness [227]. This may be of particular relevance when evaluating OS tumors that exist in the bone—a relatively rigid environment—but eventually expand into the surrounding soft tissues, which are substantially less rigid. It may also account for some of the differences in OS metastatic patterns as more than 85% of metastatic OS occurs in the lungs, a soft tissue, compared to only 21% that occurs in the bone [228].
This entry is adapted from the peer-reviewed paper 10.3390/biom13020398
References
- Rojas, G.A.; Hubbard, A.K.; Diessner, B.J.; Ribeiro, K.B.; Spector, L.G. International Trends in Incidence of Osteosarcoma (1988–2012). Int. J. Cancer 2021, 149, 1044–1053.
- American Cancer Society Key Statistics for Osteosarcoma. Available online: https://www.cancer.org/cancer/osteosarcoma/about/key-statistics.html (accessed on 12 September 2021).
- Sadykova, L.R.; Ntekim, A.I.; Muyangwa-Semenova, M.; Rutland, C.S.; Jeyapalan, J.N.; Blatt, N.; Rizvanov, A.A. Epidemiology and Risk Factors of Osteosarcoma. Cancer Investig. 2020, 38, 259–269.
- American Cancer Society Survival Rates for Osteosarcoma. Available online: https://www.cancer.org/cancer/osteosarcoma/detection-diagnosis-staging/survival-rates.html (accessed on 11 September 2021).
- Marko, T.A.; Diessner, B.J.; Spector, L.G. Prevalence of Metastasis at Diagnosis of Osteosarcoma: An International Comparison. Pediatr. Blood Cancer 2016, 63, 1006–1011.
- de Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araújo, J.M.G.; Fernandes, J.V. Biology and Pathogenesis of Human Osteosarcoma (Review). Oncol. Lett. 2020, 19, 1099–1116.
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone Sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1520–1536.
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results Program-Based Analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118.
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A Multi-tool for Tumor Progression. EMBO J. 2021, 40, e108647.
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84.
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; Lebleu, V.S.; Kalluri, R. Epithelial-to-Mesenchymal Transition Is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature 2015, 527, 525–530.
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; el Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-Mesenchymal Transition Is Not Required for Lung Metastasis but Contributes to Chemoresistance. Nature 2015, 527, 472–476.
- Lourenco, A.R.; Ban, Y.; Crowley, M.J.; Lee, S.B.; Ramchandani, D.; Du, W.; Elemento, O.; George, J.T.; Jolly, M.K.; Levine, H.; et al. Differential Contributions of Pre- And Post-EMT Tumor Cells in Breast Cancer Metastasis. Cancer Res. 2020, 80, 163–169.
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873.
- Grande, M.T.; Sánchez-Laorden, B.; López-Blau, C.; de Frutos, C.A.; Boutet, A.; Arévalo, M.; Rowe, R.G.; Weiss, S.J.; López-Novoa, J.M.; Nieto, M.A. Snail1-Induced Partial Epithelial-to-Mesenchymal Transition Drives Renal Fibrosis in Mice and Can Be Targeted to Reverse Established Disease. Nat. Med. 2015, 21, 989–997.
- Dhasarathy, A.; Phadke, D.; Mav, D.; Shah, R.R.; Wade, P.A. The Transcription Factors Snail and Slug Activate the Transforming Growth Factor-Beta Signaling Pathway in Breast Cancer. PLoS ONE 2011, 6, e26514.
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196.
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480.
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J. v Expression of Wnt3 Activates Wnt/β-Catenin Pathway and Promotes EMT-like Phenotype in Trastuzumab-Resistant HER2-Overexpressing Breast Cancer Cells. Mol. Cancer Res. 2012, 10, 1597–1606.
- Stemmer, V.; de Craene, B.; Berx, G.; Behrens, J. Snail Promotes Wnt Target Gene Expression and Interacts with β-Catenin. Oncogene 2008, 27, 5075–5080.
- Gauger, K.J.; Chenausky, K.L.; Murray, M.E.; Schneider, S.S. SFRP1 Reduction Results in an Increased Sensitivity to TGF-β Signaling. BMC Cancer 2011, 11, 59.
- Balsamo, J.; Arregui, C.; Leung, T.C.; Lilien, J. The Nonreceptor Protein Tyrosine Phosphatase PTP1B Binds to the Cytoplasmic Domain of N-Cadherin and Regulates the Cadherin-Actin Linkage. J. Cell Biol. 1998, 143, 523–532.
- Misiorek, J.O.; Przybyszewska-Podstawka, A.; Kałafut, J.; Paziewska, B.; Rolle, K.; Rivero-Müller, A.; Nees, M. Context Matters: Notch Signatures and Pathway in Cancer Progression and Metastasis. Cells 2021, 10, 94.
- Natsuizaka, M.; Whelan, K.A.; Kagawa, S.; Tanaka, K.; Giroux, V.; Chandramouleeswaran, P.M.; Long, A.; Sahu, V.; Darling, D.S.; Que, J.; et al. Interplay between Notch1 and Notch3 Promotes EMT and Tumor Initiation in Squamous Cell Carcinoma. Nat. Commun. 2017, 8, 1758.
- Timmerman, L.A.; Grego-Bessa, J.; Raya, A.; Bertrán, E.; Pérez-Pomares, J.M.; Díez, J.; Aranda, S.; Palomo, S.; McCormick, F.; Izpisúa-Belmonte, J.C.; et al. Notch Promotes Epithelial-Mesenchymal Transition during Cardiac Development and Oncogenic Transformation. Genes Dev. 2004, 18, 99–115.
- Matsuno, Y.; Coelho, A.L.; Jarai, G.; Westwick, J.; Hogaboam, C.M. Notch Signaling Mediates TGF-Β1-Induced Epithelial-Mesenchymal Transition through the Induction of Snai1. Int. J. Biochem. Cell Biol. 2012, 44, 776–789.
- Zavadil, J.; Cermak, L.; Soto-Nieves, N.; Böttinger, E.P. Integration of TGF-β/Smad and Jagged1/Notch Signalling in Epithelial-to-Mesenchymal Transition. EMBO J. 2004, 23, 1155–1165.
- di Domenico, M.; Giordano, A. Signal Transduction Growth Factors: The Effective Governance of Transcription and Cellular Adhesion in Cancer Invasion. Oncotarget 2017, 8, 36869–36884.
- Persad, A.; Venkateswaran, G.; Hao, L.; Garcia, M.E.; Yoon, J.; Sidhu, J.; Persad, S. Active β-Catenin Is Regulated by the PTEN/PI3 Kinase Pathway: A Role for Protein Phosphatase PP2A. Genes Cancer 2016, 7, 368–382.
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731.
- Maehara, O.; Suda, G.; Natsuizaka, M.; Ohnishi, S.; Komatsu, Y.; Sato, F.; Nakai, M.; Sho, T.; Morikawa, K.; Ogawa, K.; et al. Fibroblast Growth Factor-2-Mediated FGFR/Erk Signaling Supports Maintenance of Cancer Stem-like Cells in Esophageal Squamous Cell Carcinoma. Carcinogenesis 2017, 38, 1073–1083.
- Tashiro, E.; Henmi, S.; Odake, H.; Ino, S.; Imoto, M. Involvement of the MEK/ERK Pathway in EGF-Induced E-Cadherin down-Regulation. Biochem. Biophys. Res. Commun. 2016, 477, 801–806.
- Lo, H.-W.; Hsu, S.-C.; Xia, W.; Cao, X.; Shih, J.-Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.-C. Epidermal Growth Factor Receptor Cooperates with Signal Transducer and Activator of Transcription 3 to Induce Epithelial-Mesenchymal Transition in Cancer Cells via Up-Regulation of TWIST Gene Expression. Cancer Res. 2007, 67, 9066–9076.
- Uttamsingh, S.; Bao, X.; Nguyen, K.T.; Bhanot, M.; Gong, J.; Chan, J.L.K.; Liu, F.; Chu, T.T.; Wang, L.H. Synergistic Effect between EGF and TGF-Β1 in Inducing Oncogenic Properties of Intestinal Epithelial Cells. Oncogene 2008, 27, 2626–2634.
- Kim, J.; Kong, J.; Chang, H.; Kim, H.; Kim, A. EGF Induces Epithelial-Mesenchymal Transition through Phospho-Smad2/3-Snail Signaling Pathway in Breast Cancer Cells. Oncotarget 2016, 7, 85021–85032.
- Vichalkovski, A.; Gresko, E.; Hess, D.; Restuccia, D.F.; Hemmings, B.A. PKB/AKT Phosphorylation of the Transcription Factor Twist-1 at Ser42 Inhibits P53 Activity in Response to DNA Damage. Oncogene 2010, 29, 3554–3565.
- Hong, K.O.; Kim, J.H.; Hong, J.S.; Yoon, H.J.; Lee, J.I.; Hong, S.P.; Hong, S.D. Inhibition of Akt Activity Induces the Mesenchymal-to-Epithelial Reverting Transition with Restoring E-Cadherin Expression in KB and KOSCC-25B Oral Squamous Cell Carcinoma Cells. J. Exp. Clin. Cancer Res. 2009, 28, 28.
- Grotegut, S.; von Schweinitz, D.; Christofori, G.; Lehembre, F. Hepatocyte Growth Factor Induces Cell Scattering through MAPK/Egr-1-Mediated Upregulation of Snail. EMBO J. 2006, 25, 3534–3545.
- Ogunwobi, O.O.; Puszyk, W.; Dong, H.J.; Liu, C. Epigenetic Upregulation of HGF and C-Met Drives Metastasis in Hepatocellular Carcinoma. PLoS ONE 2013, 8, e63765.
- Sannino, G.; Marchetto, A.; Kirchner, T.; Grünewald, T.G.P. Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transition in Mesenchymal Tumors: A Paradox in Sarcomas? Cancer Res. 2017, 77, 4556–4561.
- Kahlert, U.D.; Joseph, J.V.; Kruyt, F.A.E. EMT- and MET-Related Processes in Nonepithelial Tumors: Importance for Disease Progression, Prognosis, and Therapeutic Opportunities. Mol. Oncol. 2017, 11, 860–877.
- Sato, H.; Hasegawa, T.; Abe, Y.; Sakai, H.; Hirohashi, S. Expression of E-Cadherin in Bone and Soft Tissue Sarcomas: A Possible Role in Epithelial Differentiation. Hum. Pathol. 1999, 30, 1344–1349.
- Yin, K.; Liao, Q.; He, H.; Zhong, D. Prognostic Value of Twist and E-Cadherin in Patients with Osteosarcoma. Med. Oncol. 2012, 29, 3449–3455.
- Sung, J.Y.; Park, S.Y.; Kim, J.H.; Kang, H.G.; Yoon, J.H.; Na, Y.S.; Kim, Y.N.; Park, B.K. Interferon Consensus Sequence-Binding Protein (ICSBP) Promotes Epithelial-to-Mesenchymal Transition (EMT)-like Phenomena, Cell-Motility, and Invasion via TGF-β Signaling in U2OS Cells. Cell Death Dis. 2014, 5, e1224.
- Cheng, G.; Liu, C.; Sun, X.; Zhang, L.; Liu, L.; Ouyang, J.; Li, B. Visfatin Promotes Osteosarcoma Cell Migration and Invasion via Induction of Epithelial-Mesenchymal Transition. Oncol. Rep. 2015, 34, 987–994.
- Cheng, Z.; Guo, Y.; Yang, Y.; Kan, J.; Dai, S.; Helian, M.; Li, B.; Xu, J.; Liu, C. Nitidine Chloride Suppresses Epithelial-to-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through Akt/GSK-3β/Snail Signaling Pathway. Oncol. Rep. 2016, 36, 1023–1029.
- Ru, N.; Liang, J.; Zhang, F.; Wu, W.; Wang, F.; Liu, X.; Du, Y. SPRY4 Intronic Transcript 1 Promotes Epithelial-Mesenchymal Transition Through Association with Snail1 in Osteosarcoma. DNA Cell Biol. 2016, 35, 290–295.
- Feng, Z.M.; Guo, S.M. Tim-3 Facilitates Osteosarcoma Proliferation and Metastasis through the NF-ΚB Pathway and Epithelial-Mesenchymal Transition. Genet. Mol. Res. 2016, 15, gmr7844.
- Lv, Y.F.; Dai, H.; Yan, G.; Meng, G.; Zhang, X.; Guo, Q. nan Downregulation of Tumor Suppressing STF CDNA 3 Promotes Epithelial-Mesenchymal Transition and Tumor Metastasis of Osteosarcoma by the Wnt/GSK-3β/β-Catenin/Snail Signaling Pathway. Cancer Lett. 2016, 373, 164–173.
- Chen, Y.; Zhang, K.; Li, Y.; He, Q. Estrogen-Related Receptor α Participates Transforming Growth Factor-β (TGF-β) Induced Epithelial-Mesenchymal Transition of Osteosarcoma Cells. Cell Adh. Migr. 2017, 11, 338–346.
- Zhang, Z.; Zhang, M.; Chen, Q.; Zhang, Q. Downregulation of MicroRNA-145 Promotes Epithelial-Mesenchymal Transition via Regulating Snail in Osteosarcoma. Cancer Gene Ther. 2017, 24, 83–88.
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656.
- Qiu, M.; Chen, D.; Shen, C.; Shen, J.; Zhao, H.; He, Y. Sex-Determining Region Y-Box Protein 3 Induces Epithelial-Mesenchymal Transition in Osteosarcoma Cells via Transcriptional Activation of Snail1. J. Exp. Clin. Cancer Res. 2017, 36, 46.
- Zhang, D.; Liu, S. SOX5 Promotes Epithelial-Mesenchymal Transition in Osteosarcoma via Regulation of Snail. J. Balk. Union Oncol. 2017, 22, 258–264.
- Wang, Z.; Chen, X.; Zhao, Y.; Jin, Y.; Zheng, J. G-Protein-Coupled Estrogen Receptor Suppresses the Migration of Osteosarcoma Cells via Post-Translational Regulation of Snail. J. Cancer Res. Clin. Oncol. 2019, 145, 87–96.
- Chen, Y.; Zhang, T.; Liu, X.; Li, Z.; Zhou, D.; Xu, W. Melatonin Suppresses Epithelial-to-Mesenchymal Transition in the MG-63 Cell Line. Mol. Med. Rep. 2020, 21, 1356–1364.
- Wen, J.F.; Jiang, Y.Q.; Li, C.; Dai, X.K.; Wu, T.; Yin, W.Z. LncRNA-XIST Promotes the Oxidative Stress-Induced Migration, Invasion, and Epithelial-to-Mesenchymal Transition of Osteosarcoma Cancer Cells through MiR-153-SNAI1 Axis. Cell Biol. Int. 2020, 44, 1991–2001.
- Sun, Y.; Jiang, X.; Lu, Y.; Zhu, J.; Yu, L.; Ma, B.; Zhang, Q. Oridonin Prevents Epithelial-Mesenchymal Transition and TGF-Β1-Induced Epithelial-Mesenchymal Transition by Inhibiting TGF-Β1/Smad2/3 in Osteosarcoma. Chem. Biol. Interact. 2018, 296, 57–64.
- Sharili, A.S.; Allen, S.; Smith, K.; Price, J.; McGonnell, I.M. Snail2 Promotes Osteosarcoma Cell Motility through Remodelling of the Actin Cytoskeleton and Regulates Tumor Development. Cancer Lett. 2013, 333, 170–179.
- Ishikawa, T.; Shimizu, T.; Ueki, A.; Yamaguchi, S.I.; Onishi, N.; Sugihara, E.; Kuninaka, S.; Miyamoto, T.; Morioka, H.; Nakayama, R.; et al. Twist2 Functions as a Tumor Suppressor in Murine Osteosarcoma Cells. Cancer Sci. 2013, 104, 880–888.
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Cyr61 Promotes Epithelial-Mesenchymal Transition and Tumor Metastasis of Osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 Signaling Pathway. Mol. Cancer 2014, 13, 236.
- Wang, Z.; Li, J.; Li, K.; Xu, J. SOX6 Is Downregulated in Osteosarcoma and Suppresses the Migration, Invasion and Epithelial-Mesenchymal Transition via TWIST1 Regulation. Mol. Med. Rep. 2018, 17, 6803–6811.
- Shi, D.; Wu, F.; Mu, S.; Hu, B.; Zhong, B.; Gao, F.; Qing, X.; Liu, J.; Zhang, Z.; Shao, Z. LncRNA AFAP1-AS1 Promotes Tumorigenesis and Epithelial-Mesenchymal Transition of Osteosarcoma through RhoC/ROCK1/P38MAPK/Twist1 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 375.
- Yu, X.J.; Guo, X.Z.; Li, C.; Chong, Y.; Song, T.N.; Pang, J.F.; Shao, M. SIRT1-ZEB1-Positive Feedback Promotes Epithelial-Mesenchymal Transition Process and Metastasis of Osteosarcoma. J. Cell Biochem. 2019, 120, 3727–3735.
- Feng, T.; Zhu, Z.; Jin, Y.; Wang, H.; Mao, X.; Liu, D.; Li, Y.; Lu, L.; Zuo, G. The MicroRNA 708 5p/ZEB1/EMT Axis Mediates the Metastatic Potential of Osteosarcoma. Oncol. Rep. 2020, 43, 491–502.
- Yao, H.; Hou, G.; Wang, Q.Y.; Xu, W.B.; Zhao, H.Q.; Xu, Y.C. LncRNA SPRY4-IT1 Promotes Progression of Osteosarcoma by Regulating ZEB1 and ZEB2 Expression through Sponging of MiR-101 Activity. Int. J. Oncol. 2020, 56, 85–100.
- Shen, A.; Zhang, Y.; Yang, H.; Xu, R.; Huang, G. Overexpression of ZEB1 Relates to Metastasis and Invasion in Osteosarcoma. J. Surg. Oncol. 2012, 105, 830–834.
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133.
- Lin, C.Y.; Hsieh, Y.S.; Chu, S.C.; Hsu, L.S.; Huang, S.C.; Chen, P.N. Reduction of Invasion and Cell Stemness and Induction of Apoptotic Cell Death by Cinnamomum Cassia Extracts on Human Osteosarcoma Cells. Environ. Toxicol. 2022, 37, 1261–1274.
- He, D.; Gao, J.; Zheng, L.; Liu, S.; Ye, L.; Lai, H.; Pan, B.; Pan, W.; Lou, C.; Chen, Z.; et al. TGF-β Inhibitor RepSox Suppresses Osteosarcoma via the JNK/Smad3 Signaling Pathway. Int. J. Oncol. 2021, 59, 84.
- Ma, K.; Zhang, C.; Li, W. Gamabufotalin Suppressed Osteosarcoma Stem Cells through the TGF-β/Periostin/PI3K/AKT Pathway. Chem. Biol. Interact. 2020, 331, 109275.
- Jiang, X.; Zhang, Z.; Song, C.; Deng, H.; Yang, R.; Zhou, L.; Sun, Y.; Zhang, Q. Glaucocalyxin A Reverses EMT and TGF-Β1-Induced EMT by Inhibiting TGF-Β1/Smad2/3 Signaling Pathway in Osteosarcoma. Chem. Biol. Interact. 2019, 307, 158–166.
- Dong, F.; Liu, T.; Jin, H.; Wang, W. Chimaphilin Inhibits Human Osteosarcoma Cell Invasion and Metastasis through Suppressing the TGF-Β1-Induced Epithelial-to-Mesenchymal Transition Markers via PI-3K/Akt, ERK1/2, and Smad Signaling Pathways. Can. J. Physiol. Pharmacol. 2018, 96, 1–7.
- Wang, Y.; Wang, H.; Zhou, R.; Zhong, W.; Lu, S.; Ma, Z.; Chai, Y. Baicalin Inhibits Human Osteosarcoma Cells Invasion, Metastasis, and Anoikis Resistance by Suppressing the Transforming Growth Factor-Β1-Induced Epithelial-to-Mesenchymal Transition. Anticancer Drugs 2017, 28, 581–587.
- Kang, H.M.; Park, B.S.; Kang, H.K.; Park, H.R.; Yu, S.B.; Kim, I.R. Delphinidin Induces Apoptosis and Inhibits Epithelial-to-Mesenchymal Transition via the ERK/P38 MAPK-Signaling Pathway in Human Osteosarcoma Cell Lines. Environ. Toxicol. 2018, 33, 640–649.
- Xie, C.; Liu, S.; Wu, B.; Zhao, Y.; Chen, B.; Guo, J.; Qiu, S.H.; Cao, Y.M. MiR-19 Promotes Cell Proliferation, Invasion, Migration, and EMT by Inhibiting SPRED2-Mediated Autophagy in Osteosarcoma Cells. Cell Transplant. 2020, 29, 963689720962460.
- Raimondi, L.; Gallo, A.; Cuscino, N.; de Luca, A.; Costa, V.; Carina, V.; Bellavia, D.; Bulati, M.; Alessandro, R.; Fini, M.; et al. Potential Anti-Metastatic Role of the Novel MiR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion. Int. J. Mol. Sci. 2022, 23, 705.
- Wang, Z.; Sun, X.; Bao, Y.; Mo, J.; Du, H.; Hu, J.; Zhang, X. E2F1 Silencing Inhibits Migration and Invasion of Osteosarcoma Cells via Regulating DDR1 Expression. Int. J. Oncol. 2017, 51, 1639–1650.
- Zheng, B.; Zhou, C.; Qu, G.; Ren, C.; Yan, P.; Guo, W.; Yue, B. VEGFR2 Promotes Metastasis and PD-L2 Expression of Human Osteosarcoma Cells by Activating the STAT3 and RhoA-ROCK-LIMK2 Pathways. Front. Oncol. 2020, 10, 543562.
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-Associated Macrophages Promote Lung Metastasis and Induce Epithelial-Mesenchymal Transition in Osteosarcoma by Activating the COX-2/STAT3 Axis. Cancer Lett. 2019, 440–441, 116–125.
- Huang, H.; Han, Y.; Chen, Z.; Pan, X.; Yuan, P.; Zhao, X.; Zhu, H.; Wang, J.; Sun, X.; Shi, P. ML264 Inhibits Osteosarcoma Growth and Metastasis via Inhibition of JAK2/STAT3 and WNT/β-Catenin Signalling Pathways. J. Cell Mol. Med. 2020, 24, 5652–5664.
- Hu, Y.; Luo, X.; Zhou, J.; Chen, S.; Gong, M.; Deng, Y.; Zhang, H. Piperlongumine Inhibits the Progression of Osteosarcoma by Downregulating the SOCS3/JAK2/STAT3 Pathway via MiR-30d-5p. Life Sci. 2021, 277, 119501.
- Zhang, J.; Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The Role of Notch Ligand Jagged1 in Osteosarcoma Proliferation, Metastasis, and Recurrence. J. Orthop. Surg. Res. 2021, 16, 226.
- Yu, L.; Xia, K.; Gao, T.; Chen, J.; Zhang, Z.; Sun, X.; Simões, B.M.; Eyre, R.; Fan, Z.; Guo, W.; et al. The Notch Pathway Promotes Osteosarcoma Progression through Activation of Ephrin Reverse Signaling. Mol. Cancer Res. 2019, 17, 2383–2394.
- Li, Z.; Tang, Y.; Xing, W.; Dong, W.; Wang, Z. LncRNA, CRNDE Promotes Osteosarcoma Cell Proliferation, Invasion and Migration by Regulating Notch1 Signaling and Epithelial-Mesenchymal Transition. Exp. Mol. Pathol. 2018, 104, 19–25.
- Deng, Y.; Zhao, F.; Zhang, Z.; Sun, F.; Wang, M. Long Noncoding RNA SNHG7 Promotes the Tumor Growth and Epithelial-to-Mesenchymal Transition via Regulation of MiR-34a Signals in Osteosarcoma. Cancer Biother Radiopharm. 2018, 33, 365–372.
- Dai, G.; Liu, G.; Zheng, D.; Song, Q. Inhibition of the Notch Signaling Pathway Attenuates Progression of Cell Motility, Metastasis, and Epithelial-to-Mesenchymal Transition-like Phenomena Induced by Low Concentrations of Cisplatin in Osteosarcoma. Eur. J. Pharmacol. 2021, 899, 174058.
- Ma, Y.; Ren, Y.; Han, E.Q.; Li, H.; Chen, D.; Jacobs, J.J.; Gitelis, S.; O’Keefe, R.J.; Konttinen, Y.T.; Yin, G.; et al. Inhibition of the Wnt-β-Catenin and Notch Signaling Pathways Sensitizes Osteosarcoma Cells to Chemotherapy. Biochem. Biophys. Res. Commun. 2013, 431, 274–279.
- Wang, S.; Zhang, D.; Han, S.; Gao, P.; Liu, C.; Li, J.; Pan, X. Fibulin-3 Promotes Osteosarcoma Invasion and Metastasis by Inducing Epithelial to Mesenchymal Transition and Activating the Wnt/β-Catenin Signaling Pathway. Sci. Rep. 2017, 7, 6215.
- Cao, J.; Han, X.; Qi, X.; Jin, X.; Li, X. TUG1 Promotes Osteosarcoma Tumorigenesis by Upregulating EZH2 Expression via MIR-144-3p. Int. J. Oncol. 2017, 51, 1115–1123.
- Zhang, M.; Wang, D.; Zhu, T.; Yin, R. RASSF4 Overexpression Inhibits the Proliferation, Invasion, EMT, and Wnt Signaling Pathway in Osteosarcoma Cells. Oncol. Res. 2017, 25, 83–91.
- Yu, M.; Guo, D.; Cao, Z.; Xiao, L.; Wang, G. Inhibitory Effect of MicroRNA-107 on Osteosarcoma Malignancy Through Regulation of Wnt/β-Catenin Signaling in Vitro. Cancer Investig. 2018, 36, 175–184.
- Tian, H.; Zhou, T.; Chen, H.; Li, C.; Jiang, Z.; Lao, L.; Kahn, S.A.; Duarte, M.E.L.; Zhao, J.; Daubs, M.D.; et al. Bone Morphogenetic Protein-2 Promotes Osteosarcoma Growth by Promoting Epithelial-Mesenchymal Transition (EMT) through the Wnt/β-Catenin Signaling Pathway. J. Orthop. Res. 2019, 37, 1638–1648.
- Fan, S.; Gao, X.; Chen, P.; Li, X. Carboxypeptidase E-ΔN Promotes Migration, Invasiveness, and Epithelial-Mesenchymal Transition of Human Osteosarcoma Cells via the Wnt-β-Catenin Pathway. Biochem. Cell Biol. 2019, 97, 446–453.
- Chen, X.; Zhong, L.; Li, X.; Liu, W.; Zhao, Y.; Li, J. Down-Regulation of MicroRNA-31-5p Inhibits Proliferation and Invasion of Osteosarcoma Cells through Wnt/β-Catenin Signaling Pathway by Enhancing AXIN1. Exp. Mol. Pathol. 2019, 108, 32–41.
- Liu, Q.; Wang, Z.; Zhou, X.; Tang, M.; Tan, W.; Sun, T.; Deng, Y. MiR-342-5p Inhibits Osteosarcoma Cell Growth, Migration, Invasion, and Sensitivity to Doxorubicin through Targeting Wnt7b. Cell Cycle 2019, 18, 3325–3336.
- Zeng, Q.; Li, Z.; Zhao, X.; Guo, L.; Yu, C.; Qin, J.; Zhang, S.; Zhang, Y.; Yang, X. Ubiquitin-Specific Protease 7 Promotes Osteosarcoma Cell Metastasis by Inducing Epithelial-Mesenchymal Transition. Oncol. Rep. 2019, 41, 543–551.
- Ding, Q.; Mo, F.; Cai, X.; Zhang, W.; Wang, J.; Yang, S.; Liu, X. LncRNA CRNDE Is Activated by SP1 and Promotes Osteosarcoma Proliferation, Invasion, and Epithelial-Mesenchymal Transition via Wnt/β-Catenin Signaling Pathway. J. Cell Biochem. 2020, 121, 3358–3371.
- Chen, X.; Zhao, W.; Fan, W. Long Non-Coding RNA GHET1 Promotes Osteosarcoma Development and Progression via Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2020, 44, 349–359.
- Yi, Z.; Pu, Y.; Gou, R.; Chen, Y.; Ren, X.; Liu, W.; Dong, P. Silencing of RIPK4 Inhibits Epithelial-Mesenchymal Transition by Inactivating the Wnt/β-Catenin Signaling Pathway in Osteosarcoma. Mol. Med. Rep. 2020, 21, 1154–1162.
- Wang, H.; Zhang, P. LncRNA-CASC15 Promotes Osteosarcoma Proliferation and Metastasis by Regulating Epithelial-Mesenchymal Transition via the Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2021, 45, 76.
- Liang, K.; Liao, L.; Liu, Q.; Ouyang, Q.; Jia, L.; Wu, G. MicroRNA-377-3p Inhibits Osteosarcoma Progression by Targeting CUL1 and Regulating Wnt/β-Catenin Signaling Pathway. Clin. Transl. Oncol. 2021, 23, 2350–2357.
- Zhang, H.; Zhou, Q.; Shen, W. Circ-FOXM1 Promotes the Proliferation, Migration and EMT Process of Osteosarcoma Cells through FOXM1-Mediated Wnt Pathway Activation. J. Orthop. Surg. Res. 2022, 17, 344.
- Bi, W.; Yang, M.; Xing, P.; Huang, T. MicroRNA MiR-331-3p Suppresses Osteosarcoma Progression via the Bcl-2/Bax and Wnt/β-Catenin Signaling Pathways and the Epithelial-Mesenchymal Transition by Targeting N-Acetylglucosaminyltransferase I (MGAT1). Bioengineered 2022, 13, 14159–14174.
- Singla, A.; Wang, J.; Yang, R.; Geller, D.S.; Loeb, D.M.; Hoang, B.H. Wnt Signaling in Osteosarcoma. In Current Advances in Osteosarcoma; Kleinerman, E.S., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 125–139. ISBN 978-3-319-04843-7.
- Zhang, D.; Wang, S.; Chen, J.; Liu, H.; Lu, J.; Hua, J.; Huang, A.; Chen, Y. Fibulin-4 Promotes Osteosarcoma Invasion and Metastasis by Inducing Epithelial to Mesenchymal Transition via the PI3K/Akt/MTOR Pathway. Int. J. Oncol. 2017, 50, 1513–1530.
- Zhang, X.; Qu, P.; Zhao, H.; Zhao, T.; Cao, N. COX-2 Promotes Epithelial-Mesenchymal Transition and Migration in Osteosarcoma MG-63 Cells via PI3K/AKT/NF-ΚB Signaling. Mol. Med. Rep. 2019, 20, 3811–3819.
- Zhang, Y.; Cheng, H.; Li, W.; Wu, H.; Yang, Y. Highly-Expressed P2X7 Receptor Promotes Growth and Metastasis of Human HOS/MNNG Osteosarcoma Cells via PI3K/Akt/GSK3β/β-Catenin and MTOR/HIF1α/VEGF Signaling. Int. J. Cancer 2019, 145, 1068–1082.
- Wang, S.; Zhao, G.; Zhao, S.; Qiao, Y.; Yang, H. The Effects of Interleukin-33 (IL-33) on Osteosarcoma Cell Viability, Apoptosis, and Epithelial-Mesenchymal Transition Are Mediated through the PI3K/AKT Pathway. Med. Sci. Monit. 2020, 26, e920766-1–e920766-10.
- Liu, W.; Jiang, D.; Gong, F.; Huang, Y.; Luo, Y.; Rong, Y.; Wang, J.; Ge, X.; Ji, C.; Fan, J.; et al. MiR-210-5p Promotes Epithelial–Mesenchymal Transition by Inhibiting PIK3R5 Thereby Activating Oncogenic Autophagy in Osteosarcoma Cells. Cell Death Dis. 2020, 11, 93.
- Lu, D.-G.; Tang, Q.-L.; Wei, J.-H.; He, F.-Y.; Lu, L.; Tang, Y.-J. Targeting EZH2 by MicroRNA-449a Inhibits Osteosarcoma Cell Proliferation, Invasion and Migration via Regulation of PI3K/AKT Signaling Pathway and Epithelial-Mesenchymal Transition. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1656–1665.
- Xing, S.; Wang, C.; Tang, H.; Guo, J.; Liu, X.; Yi, F.; Liu, G.; Wu, X. Down-Regulation of PDGFRβ Suppresses Invasion and Migration in Osteosarcoma Cells by Influencing Epithelial–Mesenchymal Transition. FEBS Open Bio. 2020, 10, 1748–1757.
- Wang, X.; Bian, Z.; Hou, C.; Li, M.; Jiang, W.; Zhu, L. Neuropilin and Tolloid-like 2 Regulates the Progression of Osteosarcoma. Gene 2021, 768, 145292.
- Zheng, J.; Liu, C.; Shi, J.; Wen, K.; Wang, X. AIM2 Inhibits the Proliferation, Invasion and Migration, and Promotes the Apoptosis of Osteosarcoma Cells by Inactivating the PI3K/AKT/MTOR Signaling Pathway. Mol. Med. Rep. 2022, 25, 53.
- Lu, K.H.; Chen, P.N.; Hsieh, Y.H.; Lin, C.Y.; Cheng, F.Y.; Chiu, P.C.; Chu, S.C.; Hsieh, Y.S. 3-Hydroxyflavone Inhibits Human Osteosarcoma U2OS and 143B Cells Metastasis by Affecting EMT and Repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 Pathways and Reduces 143B Tumor Growth in Vivo. Food Chem. Toxicol. 2016, 97, 177–186.
- Zhang, F.; Yan, T.; Guo, W.; Sun, K.; Wang, S.; Bao, X.; Liu, K.; Zheng, B.; Zhang, H.; Ren, T. Novel Oncogene COPS3 Interacts with Beclin1 and Raf-1 to Regulate Metastasis of Osteosarcoma through Autophagy. J. Exp. Clin. Cancer Res. 2018, 37, 135.
- Lv, D.-B.; Zhang, J.-Y.; Gao, K.; Yu, Z.-H.; Sheng, W.-C.; Yang, G.; Gao, Y.-Z. MicroRNA-765 Targets MTUS1 to Promote the Progression of Osteosarcoma via Mediating ERK/EMT Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4618–4628.
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein Inhibits Cell Development, Metastasis and EMT and Induces Apoptosis by Regulating ERK Signaling Pathway in Osteosarcoma. J. Recept. Signal. Transduct. 2020, 40, 49–57.
- Greenfield, E.M.; Collier, C.D.; Getty, P.J. Receptor Tyrosine Kinases in Osteosarcoma: 2019 Update. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1258, pp. 141–155.
- U.S. National Library of Medicine List of Clinical Trials Studying Tyrosine Kinase Inhibition in Osteosarcoma. Available online: https://clinicaltrials.gov/ct2/results?cond=osteosarcoma&term=tyrosine+kinase (accessed on 5 February 2023).
- Sun, W.; Wang, W.; Lei, J.; Li, H.; Wu, Y. Actin-like Protein 6A Is a Novel Prognostic Indicator Promoting Invasion and Metastasis in Osteosarcoma. Oncol. Rep. 2017, 37, 2405–2417.
- Dai, F.; Luo, F.; Zhou, R.; Zhou, Q.; Xu, J.; Zhang, Z.; Xiao, J.; Song, L. Calponin 3 Is Associated with Poor Prognosis and Regulates Proliferation and Metastasis in Osteosarcoma. Aging 2020, 12, 14037–14049.
- Wu, Y.; Zhou, W.; Yang, Z.; Li, J.; Jin, Y. MiR-185-5p Represses Cells Growth and Metastasis of Osteosarcoma via Targeting Cathepsin E. Int. J. Toxicol. 2022, 41, 115–125.
- Pang, X.; Yin, P.; Han, J.; Wang, Z.; Zheng, F.; Chen, X. CPLA2a Correlates with Metastasis and Poor Prognosis of Osteosarcoma by Facilitating Epithelial-Mesenchymal Transition. Pathol. Res. Pract. 2019, 215, 152398.
- Zhao, X.; Li, R.; Wang, Q.; Wu, M.; Wang, Y. Overexpression of Carboxypeptidase X M14 Family Member 2 Predicts an Unfavorable Prognosis and Promotes Proliferation and Migration of Osteosarcoma. Diagn. Pathol. 2019, 14, 118.
- Li, X.; Xu, R.; Liu, H.; Fang, K. CUL4A Expression in Pediatric Osteosarcoma Tissues and Its Effect on Cell Growth in Osteosarcoma Cells. Tumor Biol. 2016, 37, 8139–8144.
- Ma, Y.; Xu, X.; Luo, M. CXCR6 Promotes Tumor Cell Proliferation and Metastasis in Osteosarcoma through the Akt Pathway. Cell Immunol. 2017, 311, 80–85.
- Habel, N.; Stefanovska, B.; Carène, D.; Patiño-Garcia, A.; Lecanda, F.; Fromigué, O. CYR61 Triggers Osteosarcoma Metastatic Spreading via an IGF1Rβ-Dependent EMT-like Process. BMC Cancer 2019, 19, 62.
- Yuan, X.; Piao, L.; Wang, L.; Han, X.; Tong, L.; Shao, S.; Xu, X.; Zhuang, M.; Liu, Z. Erythrocyte Membrane Protein Band 4.1-like 3 Inhibits Osteosarcoma Cell Invasion through Regulation of Snai1-Induced Epithelial-to-Mesenchymal Transition. Aging 2020, 13, 1947–1961.
- Yang, Y.; Chen, J.; Chen, Q. Upregulation of HOXB7 Promotes Proliferation and Metastasis of Osteosarcoma Cells. Mol. Med. Rep. 2017, 16, 2773–2778.
- Xu, W.; Chen, C.; Xu, R.; Li, Y.; Hu, R.; Li, Z.; Zhu, X. Knockdown of HuR Represses Osteosarcoma Cells Migration, Invasion and Stemness through Inhibition of YAP Activation and Increases Susceptibility to Chemotherapeutic Agents. Biomed. Pharmacother. 2018, 102, 587–593.
- Gong, X.; Zheng, X.; Huang, Y.; Song, W.; Chen, G.; Chen, T. Monoacylglycerol Lipase (MAGL) Inhibition Impedes the Osteosarcoma Progression by Regulating Epithelial Mesenchymal Transition. Tohoku J. Exp. Med. 2022, 256, 19–26.
- Tang, J.; Shen, L.; Yang, Q.; Zhang, C. Overexpression of Metadherin Mediates Metastasis of Osteosarcoma by Regulating Epithelial-Mesenchymal Transition. Cell Prolif. 2014, 47, 427–434.
- Jiang, L.; Jiang, S.; Zhou, W.; Huang, J.; Lin, Y.; Long, H.; Luo, Q. Oxidized Low Density Lipoprotein Receptor 1 Promotes Lung Metastases of Osteosarcomas through Regulating the Epithelial-Mesenchymal Transition. J. Transl. Med. 2019, 17, 369.
- Zhai, Q.; Qin, J.; Jin, X.; Sun, X.; Wang, L.; Du, W.; Li, T.; Xiang, X. PADI4 Modulates the Invasion and Migration of Osteosarcoma Cells by Down-Regulation of Epithelial-Mesenchymal Transition. Life Sci. 2020, 256, 117968.
- Niinaka, Y.; Harada, K.; Fujimuro, M.; Oda, M.; Haga, A.; Hosoki, M.; Uzawa, N.; Arai, N.; Yamaguchi, S.; Yamashiro, M.; et al. Silencing of Autocrine Motility Factor Induces Mesenchymal-to-Epithelial Transition and Suppression of Osteosarcoma Pulmonary Metastasis. Cancer Res. 2010, 70, 9483–9493.
- Ren, T.; Zheng, B.; Huang, Y.; Wang, S.; Bao, X.; Liu, K.; Guo, W. Osteosarcoma Cell Intrinsic PD-L2 Signals Promote Invasion and Metastasis via the RhoA-ROCK-LIMK2 and Autophagy Pathways. Cell Death Dis. 2019, 10, 261.
- Wang, X.; Liang, X.; Liang, H.; Wang, B. SENP1/HIF-1α Feedback Loop Modulates Hypoxia-Induced Cell Proliferation, Invasion, and EMT in Human Osteosarcoma Cells. J. Cell Biochem. 2018, 119, 1819–1826.
- Yang, P.; Liu, Y.; Qi, Y.C.; Lian, Z.H. High SENP3 Expression Promotes Cell Migration, Invasion, and Proliferation by Modulating DNA Methylation of E-Cadherin in Osteosarcoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820956988.
- Meng, Q.; Ren, C.; Wang, L.; Zhao, Y.; Wang, S. Knockdown of ST6Gal-I Inhibits the Growth and Invasion of Osteosarcoma MG-63 Cells. Biomed. Pharmacother. 2015, 72, 172–178.
- Zhou, Y.; Jin, Q.; Xiao, W.; Sun, C. Tankyrase1 Antisense Oligodeoxynucleotides Suppress the Proliferation, Migration and Invasion through Hippo/YAP Pathway in Human Osteosarcoma Cells. Pathol. Res. Pract. 2019, 215, 152381.
- Zeng, S.X.; Cai, Q.C.; Guo, C.H.; Zhi, L.Q.; Dai, X.; Zhang, D.F.; Ma, W. High Expression of TRIM29 (ATDC) Contributes to Poor Prognosis and Tumor Metastasis by Inducing Epithelial-Mesenchymal Transition in Osteosarcoma. Oncol. Rep. 2017, 38, 1645–1654.
- Chen, Y.; Guo, Y.; Yang, H.; Shi, G.; Xu, G.; Shi, J.; Yin, N.; Chen, D. TRIM66 Overexpresssion Contributes to Osteosarcoma Carcin¬ Ogenesis and Indicates Poor Survival Outcome. Oncotarget 2015, 6, 23708.
- Liu, W.; Qiao, R.H.; Wang, D.M.; Huang, X.W.; Li, B.; Wang, D. UHRF1 Promotes Human Osteosarcoma Cell Invasion by Downregulating the Expression of E-Cadherin in an Rb1-Dependent Manner. Mol. Med. Rep. 2016, 13, 315–320.
- Song, C.; Liu, W.; Li, J. USP17 Is Upregulated in Osteosarcoma and Promotes Cell Proliferation, Metastasis, and Epithelial-Mesenchymal Transition through Stabilizing SMAD4. Tumor Biol. 2017, 39, 1010428317717138.
- Zhang, D.; Jiang, F.; Wang, X.; Li, G. Downregulation of Ubiquitin-Specific Protease 22 Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition in Osteosarcoma Cells. Oncol. Res. 2017, 25, 743–751.
- Xu, N.; Wang, L.; Sun, P.; Xu, S.; Fu, S.; Sun, Z. Low Arid1a Expression Correlates with Poor Prognosis and Promotes Cell Proliferation and Metastasis in Osteosarcoma. Pathol. Oncol. Res. 2019, 25, 875–881.
- Liu, P.; Yang, P.; Zhang, Z.; Liu, M.; Hu, S. Ezrin/NF-ΚB Pathway Regulates EGF-Induced Epithelial-Mesenchymal Transition (EMT), Metastasis, and Progression of Osteosarcoma. Med. Sci. Monit. 2018, 24, 2098–2108.
- Yu, G.-H.; Fu, L.; Chen, J.; Wei, F.; Shi, W.-X. Decreased Expression of Ferritin Light Chain in Osteosarcoma and Its Correlation with Epithelial-Mesenchymal Transition. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2580–2587.
- Zhang, J.; Zhang, Y.; Cheng, S.; Mu, Y.; Liu, Y.; Yi, X.; Jiang, D.; Ding, Y.; Zhuang, R. LAIR-1 Overexpression Inhibits Epithelial-Mesenchymal Transition in Osteosarcoma via GLUT1-Related Energy Metabolism. World J. Surg. Oncol. 2020, 18, 136.
- Gao, K.; Yin, J.; Dong, J. Deregulated WWOX Is Involved in a Negative Feedback Loop with MicroRNA-214-3p in Osteosarcoma. Int. J. Mol. Med. 2016, 38, 1850–1856.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674.
- Fedele, V.; Melisi, D. Permissive State of EMT: The Role of Immune Cell Compartment. Front. Oncol. 2020, 10, 587.
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Angela Nieto, M. The Transcription Factor Snail Controls Epithelial-Mesenchymal Transitions by Repressing E-Cadherin Expression. Nat. Cell Biol. 2000, 2, 76–83.
- Cech, T.R.; Steitz, J.A. The Noncoding RNA Revolution—Trashing Old Rules to Forge New Ones. Cell 2014, 157, 77–94.
- Xu, S.; Gong, Y.; Yin, Y.; Xing, H.; Zhang, N. The Multiple Function of Long Noncoding RNAs in Osteosarcoma Progression, Drug Resistance and Prognosis. Biomed. Pharmacother. 2020, 127, 110141.
- Lietz, C.E.; Garbutt, C.; Barry, W.T.; Deshpande, V.; Chen, Y.L.; Lozano-Calderon, S.A.; Wang, Y.; Lawney, B.; Ebb, D.; Cote, G.M.; et al. MicroRNA-MRNA Networks Define Translatable Molecular Outcome Phenotypes in Osteosarcoma. Sci. Rep. 2020, 10, 4409.
- Xiong, W.; Zhang, Y.; Yu, H. Comprehensive Characterization of Circular RNAs in Osteosarcoma Cell Lines. Cell. Signal. 2020, 71, 109603.
- Zhang, Y.; Li, J.; Wang, Y.; Jing, J.; Li, J. The Roles of Circular RNAs in Osteosarcoma. Med. Sci. Monit. 2019, 25, 6378–6382.
- Liu, F.; Xing, L.; Zhang, X.; Zhang, X. A Four-Pseudogene Classifier Identified by Machine Learning Serves as a Novel Prognostic Marker for Survival of Osteosarcoma. Genes 2019, 10, 414.
- Zhang, Y.; Liu, Z.; Fu, Q.; Wang, X.; Yang, L. Identification of 9-Gene Epithelial-Mesenchymal Transition Related Signature of Osteosarcoma by Integrating Multi Cohorts. Technol. Cancer Res. Treat. 2020, 19, 1533033820980769.
- Feng, Z.H.; Zheng, L.; Yao, T.; Tao, S.; Wei, X.A.; Zheng, Z.Y.; Zheng, B.J.; Zhang, X.Y.; Huang, B.; Liu, J.H.; et al. EIF4A3-Induced Circular RNA PRKAR1B Promotes Osteosarcoma Progression by MiR-361-3p-Mediated Induction of FZD4 Expression. Cell Death Dis. 2021, 12, 1025.
- Sun, F.; Yu, Z.; Wu, B.; Zhang, H.; Ruan, J. LINC00319 Promotes Osteosarcoma Progression by Regulating the MiR-455-3p/NFIB Axis. J. Gene Med. 2020, 22, e3248.
- Wu, S.; Gu, Z.; Wu, Y.; Wu, W.; Mao, B.; Zhao, S. LINC00324 Accelerates the Proliferation and Migration of Osteosarcoma through Regulating WDR66. J. Cell Physiol. 2020, 235, 339–348.
- Lian, H.; Xie, P.; Yin, N.; Zhang, J.; Zhang, X.; Li, J.; Zhang, C. Linc00460 Promotes Osteosarcoma Progression via MiR-1224-5p/FADS1 Axis. Life Sci. 2019, 233, 116757.
- Bian, X.; Sun, Y.M.; Wang, L.M.; Shang, Y.L. ELK1-Induced Upregulation LncRNA LINC02381 Accelerates the Osteosarcoma Tumorigenesis through Targeting CDCA4 via Sponging MiR-503–5p. Biochem. Biophys. Res. Commun. 2021, 548, 112–119.
- Han, G.; Guo, Q.; Ma, N.; Bi, W.; Xu, M.; Jia, J.; Wang, W. LncRNA BCRT1 Facilitates Osteosarcoma Progression via Regulating MiR-1303/FGF7 Axis. Aging 2021, 13, 15501–15510.
- Yan, L.; Wu, X.; Yin, X.; Du, F.; Liu, Y.; Ding, X. LncRNA CCAT2 Promoted Osteosarcoma Cell Proliferation and Invasion. J. Cell Mol. Med. 2018, 22, 2592–2599.
- Zhang, H.; Lin, J.; Chen, J.; Gu, W.; Mao, Y.; Wang, H.; Zhang, Y.; Liu, W. DDX11-AS1 Contributes to Osteosarcoma Progression via Stabilizing DDX11. Life Sci. 2020, 254, 117392.
- Wang, Y.; Zhao, Z.; Zhang, S.; Li, Z.; Li, D.; Yang, S.; Zhang, H.; Zeng, X.; Liu, J. LncRNA FAL1 Is a Negative Prognostic Biomarker and Exhibits Pro-Oncogenic Function in Osteosarcoma. J. Cell Biochem. 2018, 119, 8481–8489.
- Yang, W.; Shan, Z.; Zhou, X.; Peng, L.; Zhi, C.; Chai, J.; Liu, H.; Yang, J.; Zhang, Z. Knockdown of LncRNA GHET1 Inhibits Osteosarcoma Cells Proliferation, Invasion, Migration and EMT in Vitro and in Vivo. Cancer Biomark. 2018, 23, 589–601.
- Zhao, W.; Li, L. SP1-Induced Upregulation of Long Non-Coding RNA HCP5 Promotes the Development of Osteosarcoma. Pathol. Res. Pract. 2019, 215, 439–445.
- Cai, L.; Lv, J.; Zhang, Y.; Li, J.; Wang, Y.; Yang, H. The LncRNA HNF1A-AS1 Is a Negative Prognostic Factor and Promotes Tumorigenesis in Osteosarcoma. J. Cell Mol. Med. 2017, 21, 2654–2662.
- Lin, H.; Zhao, Z.; Hao, Y.; He, J.; He, J. Long Noncoding RNA HIF1A-AS2 Facilitates Cell Survival and Migration by Sponging MiR-33b-5p to Modulate SIRT6 Expression in Osteosarcoma. Biochem. Cell Biol. 2020, 98, 284–292.
- Wang, Y.; Zhang, R.; Cheng, G.; Xu, R.; Han, X. Long Non-Coding RNA HOXA-AS2 Promotes Migration and Invasion by Acting as a CeRNA of MiR-520c-3p in Osteosarcoma Cells. Cell Cycle 2018, 17, 1637–1648.
- He, J.W.; Li, D.; Zhou, J.H.; Zhu, Y.L.; Yu, B. qing SP1-Mediated Upregulation of LncRNA LMCD1-AS1 Functions a CeRNA for MiR-106b-5p to Facilitate Osteosarcoma Progression. Biochem. Biophys. Res. Commun. 2020, 526, 670–677.
- Li, J.; Wu, Q.M.; Wang, X.Q.; Zhang, C.Q. Long Noncoding RNA MiR210HG Sponges MiR-503 to Facilitate Osteosarcoma Cell Invasion and Metastasis. DNA Cell Biol. 2017, 36, 1117–1125.
- Wu, F.; Zhong, Y.; Lang, X.-B.; Tu, Y.-L.; Sun, S.-F. MNX1-AS1 Accelerates the Epithelial-Mesenchymal Transition in Osteosarcoma Cells by Activating MNX1 as a Functional Oncogene. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8194–8202.
- Zhang, L.; Zhao, G.; Ji, S.; Yuan, Q.; Zhou, H. Downregulated Long Non-Coding RNA MSC-AS1 Inhibits Osteosarcoma Progression and Increases Sensitivity to Cisplatin by Binding to MicroRNA-142. Med. Sci. Monit. 2020, 26, e921594-1–e921594-15.
- Tan, H.; Zhao, L. LncRNA Nuclear-Enriched Abundant Transcript 1 Promotes Cell Proliferation and Invasion by Targeting MiR-186-5p/HIF-1α in Osteosarcoma. J. Cell Biochem. 2019, 120, 6502–6514.
- Liu, W.; Liu, P.; Gao, H.; Wang, X.; Yan, M. Long Non-Coding RNA PGM5-AS1 Promotes Epithelial-Mesenchymal Transition, Invasion and Metastasis of Osteosarcoma Cells by Impairing MiR-140-5p-Mediated FBN1 Inhibition. Mol. Oncol. 2020, 14, 2660–2677.
- Xun, C.; Jiang, D.; Tian, Z.; Yunus, A.; Chen, J. Long Noncoding RNA Plasmacytoma Variant Translocation Gene 1 Promotes Epithelial-Mesenchymal Transition in Osteosarcoma. J. Clin. Lab. Anal. 2021, 35, e23587.
- Tong, C.-J.; Deng, Q.-C.; Ou, D.-J.; Long, X.; Liu, H.; Huang, K. LncRNA RUSC1-AS1 Promotes Osteosarcoma Progression through Regulating the MiR-340-5p and PI3K/AKT Pathway. Aging 2021, 13, 20116–20130.
- Deng, R.; Zhang, J.; Chen, J. LncRNA SNHG1 Negatively Regulates MiRNA-101-3p to Enhance the Expression of ROCK1 and Promote Cell Proliferation, Migration and Invasion in Osteosarcoma. Int. J. Mol. Med. 2019, 43, 1157–1166.
- Huang, Y.F.; Lu, L.; Shen, H.L.; Lu, X.X. LncRNA SNHG4 Promotes Osteosarcoma Proliferation and Migration by Sponging MiR-377-3p. Mol. Genet. Genom. Med. 2020, 8, e1349.
- Zhang, J.; Ju, C.; Zhang, W.; Xie, L. LncRNA SNHG20 Is Associated with Clinical Progression and Enhances Cell Migration and Invasion in Osteosarcoma. IUBMB Life 2018, 70, 1115–1121.
- Yu, X.; Hu, L.; Li, S.; Shen, J.; Wang, D.; Xu, R.; Yang, H. Long Non-Coding RNA Taurine Upregulated Gene 1 Promotes Osteosarcoma Cell Metastasis by Mediating HIF-1α via MiR-143-5p. Cell Death Dis. 2019, 10, 280.
- Zhao, X.; Xu, Y.; Sun, X.; Ma, Y.; Zhang, Y.; Wang, Y.; Guan, H.; Jia, Z.; Li, Y.; Wang, Y. MiR-17-5p Promotes Proliferation and Epithelial-Mesenchymal Transition in Human Osteosarcoma Cells by Targeting SRC Kinase Signaling Inhibitor 1. J. Cell Biochem. 2019, 120, 5495–5504.
- Zhang, H.; Zhang, J.; Meng, F.; Zhu, H.; Yan, H.; Guo, Y.; Zhang, S. MicroRNA-93 Promotes the Tumorigenesis of Osteosarcoma by Targeting TIMP2. Biosci. Rep. 2019, 39, BSR20191237.
- Chen, J.; Yan, D.; Wu, W.; Zhu, J.; Ye, W.; Shu, Q. Micro RNA-130a Promotes the Metastasis and Epithelialmesenchymal Transition of Osteosarcoma by Targeting PTEN. Oncol. Rep. 2016, 35, 3285–3292.
- Shen, S.; Huang, K.; Wu, Y.; Ma, Y.; Wang, J.; Qin, F.; Ma, J. A MiR-135b-TAZ Positive Feedback Loop Promotes Epithelial–Mesenchymal Transition (EMT) and Tumorigenesis in Osteosarcoma. Cancer Lett. 2017, 407, 32–44.
- Yao, J.; Lin, J.; He, L.; Huang, J.; Liu, Q. TNF-α/MiR-155 Axis Induces the Transformation of Osteosarcoma Cancer Stem Cells Independent of TP53INP1. Gene 2020, 726, 144224.
- Wang, X.; Zhang, L.; Zhang, X.; Xing, C.; Liu, R.; Zhang, F. MiR-196a Promoted Cell Migration, Invasion and the Epithelial-Mesenchymal Transition by Targeting HOXA5 in Osteosarcoma. Cancer Biomark. 2020, 29, 291–298.
- Chen, Z.; Zhao, G.; Zhang, Y.; Ma, Y.; Ding, Y.; Xu, N. MiR-199b-5p Promotes Malignant Progression of Osteosarcoma by Regulating HER2. J. BUON 2018, 23, 1816–1824.
- Shi, C.; Huang, C.M.; Wang, B.; Sun, T.F.; Zhu, A.X.; Zhu, Y.C. Pseudogene MSTO2P Enhances Hypoxia-Induced Osteosarcoma Malignancy by Upregulating PD-L1. Biochem. Biophys. Res. Commun. 2020, 530, 673–679.
- Ma, L.; Zhang, L.; Guo, A.; Liu, L.C.; Yu, F.; Diao, N.; Xu, C.; Wang, D. Overexpression of FER1L4 Promotes the Apoptosis and Suppresses Epithelial-Mesenchymal Transition and Stemness Markers via Activating PI3K/AKT Signaling Pathway in Osteosarcoma Cells. Pathol. Res. Pract. 2019, 215, 152412.
- Ye, F.; Tian, L.; Zhou, Q.; Feng, D. LncRNA FER1L4 Induces Apoptosis and Suppresses EMT and the Activation of PI3K/AKT Pathway in Osteosarcoma Cells via Inhibiting MiR-18a-5p to Promote SOCS5. Gene 2019, 721, 144093.
- Ye, K.; Wang, S.; Zhang, H.; Han, H.; Ma, B.; Nan, W. Long Noncoding RNA GAS5 Suppresses Cell Growth and Epithelial–Mesenchymal Transition in Osteosarcoma by Regulating the MiR-221/ARHI Pathway. J. Cell Biochem. 2017, 118, 4772–4781.
- Shen, B.; Zhou, N.; Hu, T.; Zhao, W.; Wu, D.; Wang, S. LncRNA MEG3 Negatively Modified Osteosarcoma Development through Regulation of MiR-361-5p and FoxM1. J. Cell Physiol. 2019, 234, 13464–13480.
- Zhang, G.; Li, Y.; Liao, G.; Qiu, H. LncRNA NKILA Inhibits Invasion and Migration of Osteosarcoma Cells via NF-ΚB/Snail Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4118–4125.
- Fan, H.; Liu, T.; Tian, H.; Zhang, S. Tusc8 Inhibits the Development of Osteosarcoma by Sponging Mir-197-3p and Targeting Ehd2. Int. J. Mol. Med. 2020, 46, 1311–1320.
- Zhang, Z.; Zhao, M.; Wang, G. Upregulation of MicroRNA-7 Contributes to Inhibition of the Growth and Metastasis of Osteosarcoma Cells through the Inhibition of IGF1R. J. Cell Physiol. 2019, 234, 22195–22206.
- Jiao, Z.-H.; Wang, J.-D.; Wang, X.-J. MicroRNA-16 Suppressed the Invasion and Migration of Osteosarcoma by Directly Inhibiting RAB23. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2598–2605.
- Chen, B.; Liu, J.; Qu, J.; Song, Y.; Li, Y.; Pan, S. MicroRNA-25 Suppresses Proliferation, Migration, and Invasion of Osteosarcoma by Targeting SOX4. Tumor Biol. 2017, 39, 1010428317703841.
- Gong, H.L.; Tao, Y.; Mao, X.Z.; Song, D.Y.; You, D.; Ni, J.D. MicroRNA-29a Suppresses the Invasion and Migration of Osteosarcoma Cells by Regulating the SOCS1/NF-ΚB Signalling Pathway through Negatively Targeting DNMT3B. Int. J. Mol. Med. 2019, 44, 1219–1232.
- Waresijiang, N.; Sun, J.; Abuduaini, R.; Jiang, T.; Zhou, W.; Yuan, H. The Downregulation of MIR-125a-5p Functions as a Tumor Suppressor by Directly Targeting MMP-11 in Osteosarcoma. Mol. Med. Rep. 2016, 13, 4859–4864.
- Liu, X.; Liang, Z.; Gao, K.; Li, H.; Zhao, G.; Wang, S.; Fang, J. MicroRNA-128 Inhibits EMT of Human Osteosarcoma Cells by Directly Targeting Integrin A2. Tumor Biol. 2016, 37, 7951–7957.
- Liu, Y.; Li, Y.; Liu, J.; Wu, Y.; Zhu, Q. MicroRNA-132 Inhibits Cell Growth and Metastasis in Osteosarcoma Cell Lines Possibly by Targeting Sox4. Int. J. Oncol. 2015, 47, 1672–1684.
- Shi, Y.K.; Guo, Y.H. MiR-139-5p Suppresses Osteosarcoma Cell Growth and Invasion through Regulating DNMT1. Biochem. Biophys. Res. Commun. 2018, 503, 459–466.
- Guo, Q.; Zhang, N.; Liu, S.; Pang, Z.; Chen, Z. By Targeting TRAF6, MiR-140-3p Inhibits TGF-Β1-Induced Human Osteosarcoma Epithelial-to-Mesenchymal Transition, Migration, and Invasion. Biotechnol. Lett. 2020, 42, 2123–2133.
- Fu, Y.; Tang, Y.; Wang, J.; Guo, Z. MicroRNA-181c Suppresses the Biological Progression of Osteosarcoma via Targeting Smad7 and Regulating Transforming Growth Factor-β (TGF-β) Signaling Pathway. Med. Sci. Monit. 2019, 25, 4801–4810.
- Yang, D.; Liu, G.; Wang, K. MiR-203 Acts as a Tumor Suppressor Gene in Osteosarcoma by Regulating RAB22A. PLoS ONE 2015, 10, e0132225.
- He, F.; Fang, L.; Yin, Q. MiR-363 Acts as a Tumor Suppressor in Osteosarcoma Cells by Inhibiting PDZD2. Oncol. Rep. 2019, 41, 2729–2738.
- Zhang, Y.; Wang, F.; Wang, L.; Zhang, Q. MiR-363 Suppresses Cell Migration, Invasion, and Epithelial-Mesenchymal Transition of Osteosarcoma by Binding to NOB1. World J. Surg. Oncol. 2020, 18, 83.
- Xu, M.; Jin, H.; Xu, C.X.; Sun, B.; Song, Z.G.; Bi, W.Z.; Wang, Y. MiR-382 Inhibits Osteosarcoma Metastasis and Relapse by Targeting y Box-Binding Protein 1. Mol. Ther. 2015, 23, 89–98.
- Tan, Y.; Chen, L.; Li, S.; Hao, H.; Zhang, D. MiR-384 Inhibits Malignant Biological Behavior Such as Proliferation and Invasion of Osteosarcoma by Regulating IGFBP3. Technol. Cancer Res. Treat. 2020, 19, 1533033820909125.
- Liu, Y.; Zhang, J.; Xing, C.; Wei, S.; Guo, N.; Wang, Y. MiR-486 Inhibited Osteosarcoma Cells Invasion and Epithelial-Mesenchymal Transition by Targeting PIM1. Cancer Biomark. 2018, 23, 269–277.
- Qiu, J.; Zhang, Y.; Chen, H.; Guo, Z. MicroRNA-488 Inhibits Proliferation, Invasion and EMT in Osteosarcoma Cell Lines by Targeting Aquaporin 3. Int. J. Oncol. 2018, 53, 1493–1504.
- Wang, Y.; Lin, S.; Chen, L.; Qiu, H.; Wang, J. MicroRNA-489 Suppresses Osteosarcoma Invasion, Migration and Epithelial-to-Mesenchymal Transition by Directly Targeting NAA10. Minerva Endocrinol. 2020, 45, 150–153.
- Wang, T.; Wang, D.; Zhang, L.; Yang, P.; Wang, J.; Liu, Q.; Yan, F.; Lin, F. The TGFβ-MiR-499a-SHKBP1 Pathway Induces Resistance to EGFR Inhibitors in Osteosarcoma Cancer Stem Cell-like Cells. J. Exp. Clin. Cancer Res. 2019, 38, 226.
- Guo, X.; Zhang, J.; Pang, J.; He, S.; Li, G.; Chong, Y.; Li, C.; Jiao, Z.; Zhang, S.; Shao, M. MicroRNA-503 Represses Epithelial–Mesenchymal Transition and Inhibits Metastasis of Osteosarcoma by Targeting c-Myb. Tumor Biol. 2016, 37, 9181–9187.
- Wang, D.; Bao, F.; Teng, Y.; Li, Q.; Li, J. MicroRNA-506-3p Initiates Mesenchymal-to-Epithelial Transition and Suppresses Autophagy in Osteosarcoma Cells by Directly Targeting SPHK1. Biosci. Biotechnol. Biochem. 2019, 83, 836–844.
- Wang, X.; Li, C.; Yao, W.; Tian, Z.; Liu, Z.; Ge, H. MicroRNA-761 Suppresses Tumor Progression in Osteosarcoma via Negatively Regulating ALDH1B1. Life Sci. 2020, 262, 118544.
- di Fiore, R.; Drago-Ferrante, R.; Pentimalli, F.; di Marzo, D.; Forte, I.M.; Carlisi, D.; de Blasio, A.; Tesoriere, G.; Giordano, A.; Vento, R. Let-7d MiRNA Shows Both Antioncogenic and Oncogenic Functions in Osteosarcoma-Derived 3AB-OS Cancer Stem Cells. J. Cell Physiol. 2016, 231, 1832–1841.
- Yang, H.; Zhao, L.; Zhang, Y.; Li, F.F. A Comprehensive Analysis of Immune Infiltration in the Tumor Microenvironment of Osteosarcoma. Cancer Med. 2021, 10, 5696–5711.
- Ling, J.; Sun, Y.; Pan, J.; Wang, H.; Ma, Z.; Yin, J.; Bao, Z.; Yang, H.; Liu, L. Feedback Modulation of Endothelial Cells Promotes Epithelial-Mesenchymal Transition and Metastasis of Osteosarcoma Cells by Von Willebrand Factor Release. J. Cell Biochem. 2019, 120, 15971–15979.
- Dai, J.; Qin, L.; Chen, Y.; Wang, H.; Lin, G.; Li, X.; Liao, H.; Fang, H. Matrix Stiffness Regulates Epithelial-Mesenchymal Transition via Cytoskeletal Remodeling and MRTF-A Translocation in Osteosarcoma Cells. J. Mech. Behav. Biomed. Mater. 2019, 90, 226–238.
- Bielack, S.; Kempf-Bielack, B.; Delling, G.; Exner, G.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities of Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Coperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790.
This entry is offline, you can click here to edit this entry!
