Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Renal cell carcinoma (RCC) is the third most common genitourinary cancer accounting for approximately 180,000 deaths worldwide in 2020. Although over two-thirds of patients initially present localized disease, up to 50% of them may progress to metastatic disease. Adjuvant therapy aims to reduce the recurrence risk and improve outcomes in several types of cancers but is currently an unmet need in RCC. RCC led to the evaluation of these target therapies in an early setting with conflicting results for disease-free survival and no overall survival (OS) benefit.
- adjuvant therapy
- renal cell carcinoma
- tyrosine kinase inhibitors
1. Adjuvant Therapy in RCC
1.1. Rationale Use in RCC
The United States National Cancer Database reports a 5-year (5 y) cancer-specific survival rate of 93% in patients with tumor–node–metastasis (TNM) stage I and II kidney cancers and of 71% and 14% for stage III /IV, respectively [12].
Generally, after surgical resection, most RCC recurrences occur within 3 years [13]; however, the authors showed that, after an initial 5 y disease-free interval, approximately 5% of patients experienced a renal recurrence and 15% a distant metastasis during the successive decade [14].
Overall, recurrence is reported in approximately 40% of surgically resected patients, and this relatively high risk suggests that many localized RCC patients may have micrometastatic disease at the time of nephrectomy [4]. Despite the initial nonmetastatic stage of RCC, the significant rate of patients who develop progression after nephrectomy supports the need for adjuvant therapy [15].
After curative resection, the intent of adjuvant therapy is to eradicate micrometastatic residual disease to reduce the risk of local and distant disease recurrence, with the goal of increasing the patient’s prognosis in terms of OS and DFS in patients at high risk of recurrence [16,17].
A fundamental aspect of adjuvant treatment is to identify patients at increased risk of recurrence who can benefit from adjuvant therapy in order to spare low-risk patients from the adverse effects of therapy [16].
1.2. Risk Classification
The risk of recurrence is associated with specific disease characteristics at diagnosis. Primary tumor stage is a recognized prognostic factor, with up to 26% of patients with stage T2, about 50% of patients with stage T3, and nearly all patients with stage T4 disease reporting a recurrence after surgery [18,19]. The presence of sarcomatoid characteristics and higher tumor nuclear grade (G) are also independently associated with an increased risk of cancer recurrence [20,21].
Accordingly, in localized RCC, several validated prognostic and risk stratification models were designed for the post-surgery setting to assess the risk of relapse, such as the stage, size, grade, and necrosis staging system (SSIGN) score [22], the Leibovich scoring system, and the UCLA integrated staging system (UISS) [21,23]. In the UISS system, a score out of 180 was created to stratify patients into low-, intermediate-, and high-risk prognostic categories, combining symptoms, tumor size, histology, and pathological stage [23]. The authors clearly predicted OS and DFS regardless of histological subtype.
The use of the UISS certainly predicted 2 y and 5 y survival values irrespective of tumor histology in 76.5–86.3% of patients with non-metastatic disease, [23]. The Leibovich prognostic score was produced to evaluate the risk of developing metastatic disease; it integrates tumor size, stage, grade, histologic necrosis, and regional lymph node status into an algorithm [21] and stratifies patients into low-, intermediate-, or high-risk groups, accurately predicting metastasis-free survival. Both the UISS and Leibovich algorithms were validated, but the latter showed superior predictive accuracy [21,23]. These models are proven estimators of DFS, OS, and cancer-specific survival (CSS) and have been utilized in phase III trials of adjuvant therapy in RCC, ASSURE, and SORCE [5,7]. The SSIGN was created to predict CSS in patients with clear-cell (cc) RCC only [22].
1.3. Efficacy Outcome
In an adjuvant setting, OS is considered a gold-standard metric in clinical trials requiring studies of long duration. DFS and recurrence-free survival (RFS) are intermediate clinical endpoints and well-established surrogates for OS; both are FDA-sanctioned endpoints for colorectal and skin cancer in the adjuvant setting [24]. Considering that most patients with RCC who suffer metastatic disease die of cancer, these intermediate clinical endpoints may indicate the incidence of metastatic events and be considered a surrogate for OS in RCC as well [24].
In their meta-analysis, Harshman et al. evaluated DFS as an early clinical surrogate for OS in the adjuvant setting for localized RCC. Thirteen randomized clinical trials (RCTs) enrolling 6473 patients have been evaluated, showing only a modest relationship between 5 y DFS and 5 y OS rates (R-squared, 0.48; 95% confidence interval (CI) 0.14–0.67) and between treatment effects measured by DFS and OS hazard ratios (HRs) (R-squared, 0.44; 95% CI 0.00–0.69) [24].
A recent retrospective observational study proved a statistically significant correlation between DFS and OS (95% CI: 0.65–0.74; p < 0.001) in 643 newly diagnosed RCC patients completely resected. Recurrence in patients with intermediate–high- or high-risk RCC was related to a significantly shorter OS, resulting in a concrete positive association between DFS and OS in this population [25].
2. Rational Use of Immunotherapy in an Adjuvant Setting
The debatable clinical benefit of adjuvant TKI therapy at the cost of high adverse events incidence has led the way for the development of different perioperative strategies, such as immune checkpoint inhibitors (ICIs) designed to reinforce and enhance immune activity against cancer cells.
Monoclonal antibodies against immune checkpoints such as programmed death-1/-ligand 1 (PD-1/L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have shown impressive efficacy in the metastatic setting of RCC, changing the first-line therapeutic algorithm [29,30].
PD-L1 is expressed by immune cells under inflammatory conditions and by tumor cells as an “adaptive immune mechanism” to escape anti-tumor responses; it induces an inhibitory response to T cells by binding PD-1 expressed on T cells, generating immune tolerance toward tumor cells. PD-1 and PDL-1 checkpoint inhibitors selectively block the interaction between PD-1 and PD L-1, potentially restoring effective antitumor immunity [16]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a checkpoint receptor on cytotoxic lymphocytes that ligates with B-7 exhibited on antigen-presenting cells and suppresses T-cell proliferation [16] (Figure 1).
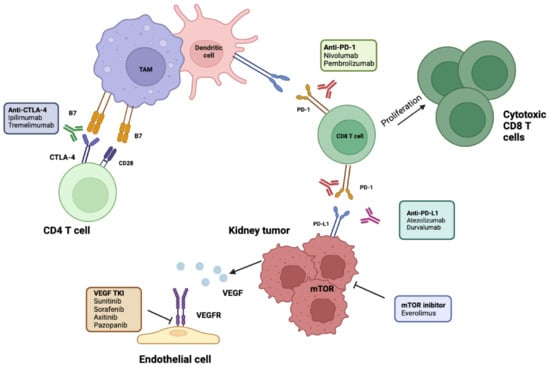
Figure 1. Molecular and immunotherapy targets evaluated in adjuvant RCC. ICIs of PD-1 disrupt its interaction with PD-L1, leading to enhanced T-cell proliferation and activation. Antibodies targeting PD-L1 prevent its interaction with PD1 on CD8 T cells, allowing their activation. Anti-CTLA-4 antibodies allow CD28 to bind to its receptor, B7, and activate naïve CD4 T cells. Antiangiogenetic targets include TKIs on VEGFR. mTOR inhibitors prevent tumor growth. TAM, tumor-associated macrophage; RCC, renal cell carcinoma; ICIs, immune checkpoint inhibitors; PD-1/L-1, programmed cell death 1/ligand-1; CTLA4, cytotoxic T-lymphocyte antigen 4; TKIs, tyrosine kinase inhibitors; VEGFR, vascular endothelial growth factor receptor, mTOR, mammalian target of rapamycin; TAM, tumor-associated macrophages. Created in BioRender.com.
Several pieces of evidence suggest that primary tumor surgical resection destroys the host’s immune system [31]. These effects lie within the “postoperative period”, which lasts days to weeks after tumor surgical resection, potentially creating an immunosuppressive window for the expansion and escape of occult tumors [31].
In preclinical and clinical metastatic settings, ICIs against PD-1 have been demonstrated to relieve postoperative T-cell dysfunction while increased T-cell activation has been shown following CTLA-4 inhibitor administration [31].
Thanks to a durable response rate and a manageable safety profile, ICIs have generated enthusiasm for the potential utility of such therapies in the adjuvant setting, as well as the maintenance of antitumor efficacy, even after their discontinuation [16,32,33].
Adjuvant Immune Checkpoint Inhibitors
KEYNOTE-564 [34] is a randomized phase III trial that evaluated pembrolizumab vs. placebo for one year in intermediate–high-risk pT2-pT3 N0, M0 RCC or high-risk (pT4, N0, M0, or any pT, any grade, N+, M0) or stage M1 with no evidence of disease (M1 NED) [34]. No prior systemic treatment for advanced RCC and disease-free survival after surgery were required. The primary endpoint was DFS by investigator assessment (INV-DFS), described as the time from randomization to the first documented relapse of RCC, secondary systemic malignancy, or death from any cause; whichever occurred first. At the first interim analysis after 30 months, the DFS was met with an HR of 0.63 (95% CI 0.50–0.80 p < 0.0001). OS was superior for the pembrolizumab arm in comparison with the placebo (HR 0.52, 95% CI 0.31–0.86, p = 0.0048). The most common adverse events (AEs) were hypertension and increased alanine aminotransferase.
In 2021, the FDA approved pembrolizumab in an adjuvant setting, which was certainly a milestone in the history of RCC treatment [35].
IMmotion010 is an ongoing randomized phase III trial aimed to assess atezolizumab, a PD-L1 inhibitor, vs placebo, for one year, in the setting of high-risk ccRCC or sarcomatoid (T2 grade 4, T3a grade 3–4, T3b/c any grade, T4 any grade, or Tx N+ any grade) after local surgery, M0, or M1NED [36]. Atezolizumab did not improve clinical outcomes in the ITT population [36]. The primary endpoint was the INV-DFS at a median follow-up of 44.7 months, which resulted in 57.2 months with atezolizumab and 49.5 months with placebo (HR 0.93, 95% CI 0.75–1.15; p = 0.50). OS was not evaluable (HR 0.97, 95% CI 0.67–1.42) [37]. Grade 3 or 4 AEs occurred in 27% and 21% of patients receiving atezolizumab or placebo, respectively.
A further ongoing ICI-adjuvant RCC trial is the Checkmate-914 phase III study, which analyzes nivolumab, an anti-PD-1 monoclonal antibody, alone or combined with ipilimumab, an anti-CTLA4 monoclonal antibody, vs placebo for 24 weeks in high-risk mostly ccRCC with pT2a (grade 3 or 4), pT2b/pT3/pT4 (any grade), N0, or any T (any grade) N1 after complete or partial nephrectomy [38]. Checkmate-914 failed the primary efficacy endpoint of INV-DFS for nivolumab with ipilimumab vs placebo (HR 0.92, 95% CI 0.71–1.19; p = 0.5347). Grade ≥ 3 treatment-related AEs were described in 28.5% vs. 2.0%, respectively. Data on nivolumab monotherapy are not currently available. The safety profile of nivolumab plus ipilimumab was in line with its known profile in advanced RCC, although a higher rate of discontinuation due to treatment-related AEs was reported with the combination vs placebo in this trial [38].
The PROSPER trial was a randomized phase III study that evaluated nivolumab in a perioperative setting for stage T2 or greater or lymph-node-positive M0 RCC of any histology [39]. Pre-surgery participants were randomized to receive two doses of neoadjuvant nivolumab succeeded by adjuvant nivolumab for 9 months or standard nephrectomy followed by observation. The trial was stopped early due to futility. The primary endpoint was RFS, which reported similar results between the two arms; OS was not mature at the time of analysis but was not statistically different between study arms. The 20% of patients treated with nivolumab experienced at least one grade 3–4 AE, compared with 6% in the control arm. Another randomized phase III trial involving ICIs is the RAMPART study, which is evaluating durvalumab, an anti-PD-L1 monoclonal antibody, plus tremelimumab, an anti-CTLA-4 monoclonal antibody, in post-surgery intermediate–high-risk RCC [40]. The primary endpoints are DFS and OS. The planned enrolment is 1750 patients.
Finally, the LITESPARK 002 was designed to compare the efficacy and safety of belzutifan, HIF-2α inhibitor, in combination with pembrolizumab vs placebo, plus pembrolizumab as an adjuvant treatment of ccRCC at intermediate–high or high risk, including M1 NED [41]. The primary endpoint is DFS, and the planned enrolment is 1600 patients [42]. The data are summarized in Table 2.
Table 2. Clinical trials on immune checkpoint inhibitors in RCC adjuvant treatment.
| Clinical Trial [Ref.] | No. of Patients | Tumor Features | Treatment Arms | Duration of Treatment | DFS | RFS | OS | Grade 3 or Worse AEs |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-564 [34] | 994 | Intermediate–high-risk M0-M1 NED Clear-cell RCC/sarcomatoid |
Pembrolizumab Placebo |
1 year | HR 0.63 (95% CI 0.50–0.80 p < 0.0001) | 75.2% (95% CI 70.8–79.1) 65·5% (60.9–69.7) |
HR 0.52 (95% CI 0.31–0.86, p = 0.0048) | 32% 18% |
| IMmotion010 [36] | 778 | Intermediate–high-risk M0-M1 NED Clear-cell RCC/sarcomatoid |
Atezolizumab Placebo |
1 year | HR 0.93 (95% CI 0.75–1.15, p = 0.50) | NA | HR 0.97 (95% CI 0.67–1.42) | 28% 24% |
| Checkmate-914 [38] | 816 | Intermediate-high risk M0 Clear-cell RCC/sarcomatoid |
Nivolumab + Ipilimumab Placebo |
At least 24 weeks | HR 0.92 (95% CI 0.71–1.20) | NA | NA | 28.5% 2% |
| PROSPER [39] | 819 | Intermediate-high risk, M0 or M1 NED RCC of any histology |
Nivolumab neoadjuvant- adjuvant Placebo |
40 weeks (One dose prior to surgery followed by 9 doses) |
NA | HR: 0.97 (95% CI: 0.74–1.28; P1-sided = 0.43) | HR: 1.48; (95% CI: 0.89–2.48; P1-sided = 0.93). | 20% 6% |
| LITESPARK 002 [41] | 1600 | Clear-cell RCC pT2, grade 4 or sarcomatoid, N0, M0 or pT3, any grade, N0, M0, high (pT4, any grade, N0, M0 or pT, any stage/grade, N+, M0) or M1 NED | Belzutifan + pembrolizumab Placebo + pembrolizumab |
1 year | DFS | NA | NA | NA |
| RAMPART [40] | 1750 | Clear-cell and non-clear-cell histological RCC subtypes with high or intermediate risk of relapse (Leibovich score 3–11). | Durvaumab + tremelimumab Placebo |
1 year | DFS and OS | NA | NA | NA |
NED, no evidence of disease; RCC, renal cell carcinoma; DFS, disease-free survival; RFS, relapse-free survival; CI, confidence interval; AEs, adverse events; OS, overall survival; NA, not available.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24044243
This entry is offline, you can click here to edit this entry!
