Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Developmental dyslexia is a common complex neurodevelopmental disorder that persists well into adulthood and, thus, could have lifelong detrimental effects. Dyslexia has been defined as “deficient literacy acquisition despite adequate intellectual ability and sufficient educational exposure”. Here we look at it from latest neuro scietiific models.
- dyslexia
- cerebellum
- neuroplasticity
- neurorehabilitation
1. Neural Correlates of Dyslexia
Given the complex neuropsychological profile of this disorder, many studies have investigated dyslexia using a different approach, namely, searching for structural and functional differences in the dyslexic brain compared to normal-reading individuals. As the most characterizing deficit of dyslexia, the phonological deficit has been found to be associated with abnormalities in cortical response and asymmetrical activity in frontal and temporal language/reading areas [1][2][3]. In particular, reduced activity has been identified in a left temporoparietal region (related to phonological processing and phoneme-grapheme conversion) and in a left occipitotemporal area (linked to whole-word recognition) (for a review, see [4]). Moreover, during reading and phonologic processing tasks, reduced blood flow in the left temporoparietal area has been found among adult dyslexics compared to normal readers in PET studies [5][6][7], while normal activation was found in left inferior frontal areas [5][6]. In addition, the enhanced asymmetrical activity in the left hemisphere observed in normal readers, evidenced by fMRI studies, for instance, in which normal readers demonstrated increasingly higher activation in temporoparietal areas in relation to increasing demands for phonological processing [8][9] is in contrast to the experience of adult dyslexic readers, in whom this activation has been found significantly attenuated during reading tasks [10].
These fronto-temporal and cerebellar functional abnormalities identified in individuals with dyslexia are further corroborated by results coming from structural imaging studies. Raschle and colleagues [11] reported decreased grey matter (GM) in the frontal and temporal networks of dyslexics, while local white matter (WM) change was demonstrated in left temporoparietal regions and in the left inferior frontal gyrus [12][13][14], areas corresponding to phonological skills (for a review, see [4]). Structural imaging also demonstrated lower cerebellar declive volume (associated with impaired reading abilities; [15]) and, as mentioned before, Eckert and colleagues [16] found the volume of the right anterior lobe of the cerebellum significantly correlated with reading performances.
The extent of the differences between the dyslexic brain and the normal reading brain is not limited to these structures. Dyslexic individuals can differ from controls across a wide array of brain areas, including the insula, caudate, corpus callosum, left temporal lobe, and thalamus [17][18][19][20][21][22][23]. As an example, the insula is thought to be involved in the auditory temporal processing of non-linguistic auditory stimuli, and the aforementioned area constitutes a neural correlate of deficit temporal processing of speech and nonspeech sounds in dyslexia [24]. Nonetheless, the cerebellar and frontal differences between dyslexics and controls are by far the most consistently reported (for reviews, see [15][25]). As Eckert concluded in his 2004 review [26], the main regions that display structural changes in dyslexia are the inferior parietal lobule, the inferior frontal gyrus and the cerebellum.
2. Cerebellar Involvement in Reading and Dyslexia
Functional imaging studies have shown activation in the left inferior frontal and right cerebellar hemispheres during fluency tasks, verb generation tasks, passive listening to clicks, linguistic working memory tasks and rapid production of verbal stimuli [27][28][29][30][31]. In addition to this evidence suggesting a functional role of the cerebellum in language processing, a wealth of studies exist supporting this link [32][33][34][35]. Moreover, a meta-analysis of the functional topography of the cerebellum identified activation in right cerebellar regions, including lobules VI and crus I/II, during language processing, thus further reinforcing the cerebellar role in language [36]. Booth and colleagues [37] also provided evidence for reciprocal functional connections between the cerebellum and the left inferior frontal and left lateral temporal regions, two areas already known to be involved in language processing. While the involvement of the cerebellum during language processing seems clear, what is its precise involvement during reading?
Reading-related activity in the cerebellum seems to be focused on lobules VI and VII, reaching their maximum in the right posterolateral cerebellum [38][39]. It is evident that these are the same cerebellar areas activated during language tasks [36]. The precise localizations of activation depend on the demands of the particular reading task, but the most consistent activation has been found in the left, the right, or bilateral lobules V, VI and VII (for a review, see [40]). Cerebellar involvement in reading is also highlighted by differences between impaired and normal readers. Normal readers usually show right-lateralized cerebellar asymmetry, while dyslexic individuals usually show symmetry that is correlated with phonological processing deficits [21][41]. Although reduced cerebellar size and asymmetry may be related to general cognitive deficits and not only to dyslexia [42], many studies demonstrated cerebellar abnormalities among dyslexic individuals. The results from Eckert and colleagues [16] echo that of Leonard and colleagues [20], who found the right anterior cerebellum smaller in dyslexic adults. Structural imaging further confirms, although not consistently [43][44], that the dyslexic brain shows significantly reduced grey matter in cerebellar nuclei, lateral lobule VII, and the anterior cerebellum [19][45][46]. Similar to the structural findings, the anterior lobe of the cerebellum has also evidenced reduced activation in dyslexics [47][48], as well as alterations in functional connectivity between this area and the angular and inferior frontal gyri [49][50], two brain regions critical to reading and phonological processes.
3. Interventions, Neuroplasticity and Dyslexia
In the next sections, examine studies investigating different types of interventions in children affected by dyslexia, the effects of sensorimotor training in dyslexic participants and linked functional and structural changes, and all the available literature existing on the QMT. Consulted databases were PsycInfo, PubMed and Google Scholar. This resulted in a total of 27 non-theoretical, non-review/meta-analysis studies being included. A cumulative sample of 241 dyslexic participants and 195 children with a familial risk of dyslexia were included. In addition, counted separately, all the examined studies on the QMT yielded a total of 608 participants (23 with dyslexia and 585 healthy participants).
Dyslexia has long been considered a chronic reading disorder in which a number of symptoms from childhood can persist into adulthood [51][52][53][54], regardless of language transparency [55][56]. Over the years, different types of interventions have been advanced, trying to find ways to reduce or eliminate symptoms of dyslexia. Traditional interventions, such as direct teaching, have garnered a solid base of evidence for their efficacy, but many alternative therapies for dyslexia exist, with different degrees of success [57]. The most effective interventions seem to be those that provide intensive and explicit instruction in phoneme awareness, reading fluency, and reading comprehension [58], thus primarily addressing the typical phonological deficits of dyslexia. Torgesen and colleagues [59][60] demonstrated that phonological problems could be remediated with relatively short periods of intensive remedial instruction. For example, the authors contrasted two different treatment approaches among dyslexic children: (1) a multisensory, bottom–up, explicit approach for developing phonemic awareness, and phonemic decoding/encoding skills with minimal text instruction and (2) and embedded phonics instruction approach. The outcome of this intensive experiment was a general improvement in reading and phonemic decoding, regardless of the type of intervention. However, while almost all children displayed better results after the intervention, only half of the outcomes remained stable or continued to improve during follow-up, and some children even lost the acquired benefits [60]. In a similar way, a study by Simos and colleagues [61] showed that all children in their cohort displayed a typical dyslexia-specific profile of weak activity in the left posterior superior temporal gyrus and inferior parietal areas, and strong activation of homologous regions in the right hemisphere in the pre-intervention phase. The authors demonstrated how completion of a phonologically based reading program resulted in consistent improvement in phonologic decoding abilities and normalization of the brain activation profiles for all of the children [61].
While such intervention programs have been phonologically focused, there is a wealth of data relating to other types of deficits. Thus, in creating, evaluating, and administering treatments, factors beyond language intervention should be considered to achieve optimal outcomes. For example, “cool” executive functions, such as attention and working memory, should be assessed and treated [62], together with “hot” executive functions and emotional aspects, such as low self-esteem, anxiety and behavioural regulation, to fully encompass the well-being of people with dyslexia [63][64][65][66][67][68][69][70]. A recent study [71] implemented a remedial technique for dyslexia the consisted of asking children to repeatedly read texts aloud while listening to a song (reading with vocal musical masking, or RVM; [72]). According to Leloup and colleagues [71], their hypothesis was that this intervention would improve executive skills rather than directly reinforcing specific coding skills, leading to improved reading abilities. Their findings indeed supported their hypothesis, that processing speed and phonemic fluency were the best predictors of improved reading skills related to the remedial intervention [71]. In a related vein, other studies examined interventions that tapped into a wider array of functions, demonstrating the role of music-making and listening as a potential tool in the remediation of dyslexia symptoms [73][74][75][76] and also having some deep effect on improving mood and elicit positive emotions (for a review on brain correlates and possible applications of music in treating neurological and psychiatric disorder see [77]). In particular, the act of practicing an instrument, which requires multi-sensory, motor integration, emotional engagement and creativity, may compensate for dyslexic deficits, such as poor working memory and accuracy during reading [74][75] eliciting changes in the central and peripheral nervous systems that correlate with improvements in motor, auditory, and learning abilities [78][79]. Such improvements related to musical training (i.e., piano lessons) were described in a case study by Eren [80] demonstrating that, after 8 months of instrument training, the dyslexic participant showed not only strengthened attention and memory, but also higher confidence, better behavioral regulation, and generally more positive emotions [80].
More generally, the link between physical activity, motor skills and dyslexia has been investigated by different authors in the last 20 years. While motor ability can be further clarified as fine and gross motor skills [81][82], which may, in turn, influence language and reading skills in different ways, here we will mostly focus on gross motor skills. For example, in 2001, Lyytinen and colleagues compared children with and without familial risk for dyslexia from birth to five years old on a series of variables pertaining to motor skills and language development. They attempted to identify potential predictor variables for dyslexia and found that early vocalization and motor development, especially gross motor skills, were linked to later language development [83]. Along similar lines, another longitudinal study highlighted how children at risk for dyslexia and with slower motor development showed a smaller vocabulary and produced shorter sentences compared to other children [84]. In addition, from also a cross-sectional perspective, dyslexic children were found to have lower performance in balance tasks compared to controls [85].
Furthermore, physical activity has been found to be linked to cognitive functioning in children with learning disabilities [86]. This meta-analysis found a significant positive relationship between physical activity and cognitive functioning, suggesting that physical activity can be beneficial both for children with learning disabilities and with healthy children, highlighting the importance of physical activity for their well-being [86].
One reason for positive outcomes using sensorimotor interventions in the remediation of dyslexic deficits and the connection between language acquisition and motor skills could lie in the aforementioned role of cerebellar circuits in phonological processing via articulatory monitoring [87][88]. For this reason, Stoodley and Stein [40] (p. 271) suggested that “cerebellar circuits may be particularly important for compensation and remediation of reading difficulties”. Consequently, studies aimed to investigate possible improvements in reading through integrated sensory stimulation and visuo-motor and vestibular exercises [89][90]. Although these results are compelling, the effectiveness of such evidence-based intervention programs is often debated [3][89][90][91][92][93].
A suggestion on how to explore multiple levels of the effects of sensorimotor training comes from a recent model presented by Ben-Soussan and colleagues [94] which outlined the importance of the cerebellar activity in higher cognitive functions and examined its influence on different levels of brain organization (Figure 2). The authors suggested the presence of two intertwined pathways leading to functional and structural changes, one related to alterations in cerebellar oscillatory activity and the other, with a longer time frame, related to neurotrophic factors and neuroplasticity [94]. Both pathways, and their main preliminary starting point (i.e., the cerebellar activity), are altered in dyslexia and other neurodevelopmental disorders [95][96][97].
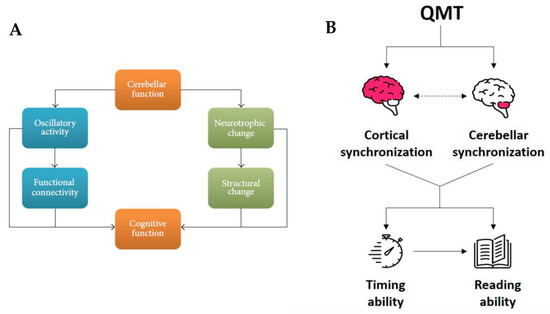
Figure 2. (A) Interconnected relationship between cerebellar and cognitive function. The relationship is mediated via two interrelated routes. The first is slow rhythm oscillations, manifested in functional connectivity; the second is molecular effects on structural changes in connectivity. Adapted from [94]. (B) Effects of the QMT on both timing and reading abilities mediated by the interconnected relationship between cortical and cerebellar synchronization. Adapted from [98].
Its focus on cerebellar involvement, neurobiological factors and the junction between functional and structural pathways makes Ben-Soussan and colleagues’ model [94] a novel alternative way to integrate both magnocellular and cerebellar theories of dyslexia. Moreover, the processes it emphasizes, which connect cerebellar to cognitive functions, could help in interpreting and understanding the complex dynamics of dyslexia’s deficits. The model is mainly based on two lines of evidence. The first relates to the functional level and the many already mentioned reports suggesting cerebellar alpha oscillatory activity as the neural system underlying the planning and execution of movements as well as reading and language comprehension [99][100][101].
The second line of evidence pertains to an anatomical and structural level, particularly focusing on Brain-Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF) from the neurotrophin family. Neurotrophins, and their precursors, pro-neurotrophins, can influence mature and developing neural circuits in different ways [102][103]. For example, ProBDNF and NGF have been linked to learning, spatial cognition and neuronal plasticity [104][105][106][107], while proNGF is related to nociceptors, neuronal death and neurodegeneration [108]. Importantly, BDNF has been found to be a crucial factor in mediating neuronal connectivity and movement-dependent plasticity in the cerebellum [109][110]. Further, among human participants, functional connectivity changes induced by sensorimotor training were found to be BDNF-dependent [111][112]. In the same way, animal models also demonstrated a relationship between BDNF and cerebellar functioning, identifying important cerebellar deficits in BDNF knockout mice [113][114]. Moreover, numerous animal models have demonstrated training-induced effects in the motor cortex, the basal ganglia and the cerebellum (for reviews, see [111][112][115][116]).
Nevertheless, human studies examining motor training-induced changes in cognitive functions have generally neglected to study motor-related brain areas. Such studies have almost exclusively concentrated on frontal and prefrontal areas, reporting an increase in frontal alpha synchronization after motor training [117][118][119]. The model by Ben-Soussan and co-workers [94] (p. 3) suggests that “motor training in humans may serve as an optimal model to test the possible cerebellar cognition relationship in two interrelated routes: (1) sensorimotor training may result in fast occurring slow frequency oscillatory modulation leading to improved cognitive performance. (2) Cerebellar changes can be further stimulated through training by activating molecular mechanisms (e.g., neurotrophins)”. Interestingly, the importance of cerebellar oscillatory function in neuroplasticity [120][121] has long been acknowledged in studies related to motor learning. A couple of studies [122][123] have already demonstrated cerebellar microstructural changes following sensorimotor training. Thus, techniques such as sensorimotor training paradigms may efficiently achieve beneficial effects in the treatment of dyslexia impairments such as the decreased right cerebellar volume reported by Eckert and colleagues among dyslexics compared to controls [16], through the dual functional and structural pathways highlighted in this model [94].
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20043315
References
- Ramus, F. Neurobiology of Dyslexia: A Reinterpretation of the Data. Trends Neurosci. 2004, 27, 720–726.
- Dufor, O.; Serniclaes, W.; Sprenger-Charolles, L.; Démonet, J.-F. Top-down Processes during Auditory Phoneme Categorization in Dyslexia: A PET Study. NeuroImage 2007, 34, 1692–1707.
- Gabrieli, J.D.E. Dyslexia: A New Synergy Between Education and Cognitive Neuroscience. Science 2009, 325, 280–283.
- Peterson, R.L.; Pennington, B.F. Developmental Dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307.
- Rumsey, J.M.; Andreason, P.; Zametkin, A.J.; Aquino, T.; King, A.C.; Hamburger, S.D.; Pikus, A.; Rapoport, J.L.; Cohen, R.M. Failure to Activate the Left Temporoparietal Cortex in Dyslexia: An Oxygen 15 Positron Emission Tomographic Study. Arch. Neurol. 1992, 49, 527–534.
- Paulesu, E.; Frith, U.; Snowling, M.; Gallagher, A.; Morton, J.; Frackowiak, R.S.J.; Frith, C.D. Is Developmental Dyslexia a Disconnection Syndrome?: Evidence from PET Scanning. Brain 1996, 119, 143–157.
- Rumsey, J.; Horwitz, B.; Donohue, B.; Nace, K.; Maisog, J.; Andreason, P. Phonological and Orthographic Components of Word Recognition. A PET-RCBF Study. Brain J. Neurol. 1997, 120, 739–759.
- Shaywitz, S.E.; Shaywitz, B.A.; Pugh, K.R.; Fulbright, R.K.; Constable, R.T.; Mencl, W.E.; Shankweiler, D.P.; Liberman, A.M.; Skudlarski, P.; Fletcher, J.M.; et al. Functional Disruption in the Organization of the Brain for Reading in Dyslexia. Proc. Natl. Acad. Sci. USA 1998, 95, 2636–2641.
- Temple, E.; Poldrack, R.A.; Salidis, J.; Deutsch, G.K.; Tallal, P.; Merzenich, M.M.; Gabrieli, J.D.E. Disrupted Neural Responses to Phonological and Orthographic Processing in Dyslexic Children: An FMRI Study. Neuroreport 2001, 12, 299–307.
- Gross-glenn, K.; Duara, R.; Barker, W.W.; Loewenstein, D.; Chang, J.-Y.; Yoshii, F.; Apicella, A.M.; Pascal, S.; Boothe, T.; Sevush, S. Positron Emission Tomographic Studies during Serial Word-Reading by Normal and Dyslexic Adults. J. Clin. Exp. Neuropsychol. 1991, 13, 531–544.
- Raschle, N.M.; Chang, M.; Gaab, N. Structural Brain Alterations Associated with Dyslexia Predate Reading Onset. NeuroImage 2011, 57, 742–749.
- Klingberg, T.; Hedehus, M.; Temple, E.; Salz, T.; Gabrieli, J.D.; Moseley, M.E.; Poldrack, R.A. Microstructure of Temporo-Parietal White Matter as a Basis for Reading Ability: Evidence from Diffusion Tensor Magnetic Resonance Imaging. Neuron 2000, 25, 493–500.
- Deutsch, G.K.; Dougherty, R.F.; Bammer, R.; Siok, W.T.; Gabrieli, J.D.E.; Wandell, B. Children’s Reading Performance Is Correlated with White Matter Structure Measured by Diffusion Tensor Imaging. Cortex 2005, 41, 354–363.
- Rimrodt, S.L.; Peterson, D.J.; Denckla, M.B.; Kaufmann, W.E.; Cutting, L.E. White Matter Microstructural Differences Linked to Left Perisylvian Language Network in Children with Dyslexia. Cortex 2010, 46, 739–749.
- Pernet, C.R.; Poline, J.B.; Demonet, J.F.; Rousselet, G.A. Brain Classification Reveals the Right Cerebellum as the Best Biomarker of Dyslexia. BMC Neurosci. 2009, 10, 67.
- Eckert, M.A.; Leonard, C.M.; Richards, T.L.; Aylward, E.H.; Thomson, J.; Berninger, V.W. Anatomical Correlates of Dyslexia: Frontal and Cerebellar Findings. Brain 2003, 126, 482–494.
- Pennington, B.F. Toward an Integrated Understanding of Dyslexia: Genetic, Neurological, and Cognitivemechanisms. Dev. Psychopathol. 1999, 11, 629–654.
- Eliez, S.; Rumsey, J.M.; Giedd, J.N.; Schmitt, J.E.; Patwardhan, A.J.; Reiss, A.L. Morphological Alteration of Temporal Lobe Gray Matter in Dyslexia: An MRI Study. J. Child Psychol. Psychiatry 2000, 41, 637–644.
- Brown, W.E.; Eliez, S.; Menon, V.; Rumsey, J.M.; White, C.D.; Reiss, A.L. Preliminary Evidence of Widespread Morphological Variations of the Brain in Dyslexia. Neurology 2001, 56, 781–783.
- Leonard, C.M.; Eckert, M.A.; Lombardino, L.J.; Oakland, T.; Kranzler, J.; Mohr, C.M.; King, W.M.; Freeman, A. Anatomical Risk Factors for Phonological Dyslexia. Cereb. Cortex 2001, 11, 148–157.
- Rae, C.; Harasty, J.A.; Dzendrowskyj, T.E.; Talcott, J.B.; Simpson, J.M.; Blamire, A.M.; Dixon, R.M.; Lee, M.A.; Thompson, C.H.; Styles, P.; et al. Cerebellar Morphology in Developmental Dyslexia. Neuropsychologia 2002, 40, 1285–1292.
- Robichon, F.; Bouchard, P.; Démonet, J.-F.; Habib, M. Developmental Dyslexia: Re-Evaluation of the Corpus Callosum in Male Adults. Eur. Neurol. 2000, 43, 233–237.
- Robichon, F.; Levrier, O.; Farnarier, P.; Habib, M. Developmental Dyslexia: Atypical Cortical Asymmetries and Functional Significance: Cortical Asymmetries in Developmental Dyslexia. Eur. J. Neurol. 2000, 7, 35–46.
- Steinbrink, C.; Ackermann, H.; Lachmann, T.; Riecker, A. Contribution of the Anterior Insula to Temporal Auditory Processing Deficits in Developmental Dyslexia. Hum. Brain Mapp. 2009, 30, 2401–2411.
- Pernet, C.; Andersson, J.; Paulesu, E.; Demonet, J.F. When All Hypotheses Are Right: A Multifocal Account of Dyslexia. Hum. Brain Mapp. 2009, 30, 2278–2292.
- Eckert, M. Neuroanatomical Markers for Dyslexia: A Review of Dyslexia Structural Imaging Studies. Neuroscientist 2004, 10, 362–371.
- Raichle, M.E.; Fiez, J.A.; Videen, T.O.; MacLeod, A.-M.K.; Pardo, J.V.; Fox, P.T.; Petersen, S.E. Practice-Related Changes in Human Brain Functional Anatomy during Nonmotor Learning. Cereb. Cortex 1994, 4, 8–26.
- Desmond, J.E.; Gabrieli, J.D.E.; Wagner, A.D.; Ginier, B.L.; Glover, G.H. Lobular Patterns of Cerebellar Activation in Verbal Working-Memory and Finger-Tapping Tasks as Revealed by Functional MRI. J. Neurosci. 1997, 17, 9675–9685.
- Schlosser, R.; Hutchinson, M.; Joseffer, S.; Rusinek, H.; Saarimaki, A.; Stevenson, J.; Dewey, S.L.; Brodie, J.D. Functional Magnetic Resonance Imaging of Human Brain Activity in a Verbal Fluency Task. J. Neurol. Neurosurg. Psychiatry 1998, 64, 492–498.
- Ackermann, H.; Riecker, A.; Mathiak, K.; Erb, M.; Grodd, W.; Wildgruber, D. Rate-Dependent Activation of a Prefrontal-Insular-Cerebellar Network during Passive Listening to Trains of Click Stimuli: An FMRI Study. Neuroreport 2001, 12, 4087–4092.
- Wildgruber, D.; Ackermann, H.; Grodd, W. Differential Contributions of Motor Cortex, Basal Ganglia, and Cerebellum to Speech Motor Control: Effects of Syllable Repetition Rate Evaluated by FMRI. NeuroImage 2001, 13, 101–109.
- Marien, P.; Engelborghs, S.; Fabbro, F.; De Deyn, P.P. The Lateralized Linguistic Cerebellum: A Review and a New Hypothesis. Brain Lang. 2001, 79, 580–600.
- Stoodley, C.J. The Cerebellum and Cognition: Evidence from Functional Imaging Studies. Cerebellum 2012, 11, 352–365.
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional Topography of the Cerebellum for Motor and Cognitive Tasks: An FMRI Study. NeuroImage 2012, 59, 1560–1570.
- Keren-Happuch, E.; Chen, S.A.; Ho, M.R.; Desmond, J.E. A Meta-analysis of Cerebellar Contributions to Higher Cognition from PET and FMRI Studies. Hum. Brain Mapp. 2014, 35, 593.
- Stoodley, C.J.; Schmahmann, J.D. The Cerebellum and Language: Evidence from Patients with Cerebellar Degeneration. Brain Lang. 2009, 110, 149–153.
- Booth, J.R.; Wood, L.; Lu, D.; Houk, J.C.; Bitan, T. The Role of the Basal Ganglia and Cerebellum in Language Processing. Brain Res. 2007, 1133, 136–144.
- Turkeltaub, P.E.; Gareau, L.; Flowers, D.L.; Zeffiro, T.A.; Eden, G.F. Development of Neural Mechanisms for Reading. Nat. Neurosci. 2003, 6, 767–773.
- Mechelli, A.; Gorno-Tempini, M.L.; Price, C.J. Neuroimaging Studies of Word and Pseudoword Reading: Consistencies, Inconsistencies, and Limitations. J. Cogn. Neurosci. 2003, 15, 260–271.
- Stoodley, C.J.; Stein, J.F. Cerebellar Function in Developmental Dyslexia. Cerebellum 2013, 12, 267–276.
- Kibby, M.Y.; Fancher, J.B.; Markanen, R.; Hynd, G.W. A Quantitative Magnetic Resonance Imaging Analysis of the Cerebellar Deficit Hypothesis of Dyslexia. J. Child Neurol. 2008, 23, 368–380.
- Leonard, C.M.; Kuldau, J.M.; Maron, L.; Ricciuti, N.; Mahoney, B.; Bengtson, M.; DeBose, C. Identical Neural Risk Factors Predict Cognitive Deficit in Dyslexia and Schizophrenia. Neuropsychology 2008, 22, 147–158.
- Silani, G.; Frith, U.; Demonet, J.-F.; Fazio, F.; Perani, D.; Price, C.; Frith, C.D.; Paulesu, E. Brain Abnormalities Underlying Altered Activation in Dyslexia: A Voxel Based Morphometry Study. Brain 2005, 128, 2453–2461.
- Hoeft, F.; Meyler, A.; Hernandez, A.; Juel, C.; Taylor-Hill, H.; Martindale, J.L.; McMillon, G.; Kolchugina, G.; Black, J.M.; Faizi, A.; et al. Functional and Morphometric Brain Dissociation between Dyslexia and Reading Ability. Proc. Natl. Acad. Sci. USA 2007, 104, 4234–4239.
- Brambati, S.M.; Termine, C.; Ruffino, M.; Stella, G.; Fazio, F.; Cappa, S.F.; Perani, D. Regional Reductions of Gray Matter Volume in Familial Dyslexia. Neurology 2004, 63, 742–745.
- Kronbichler, M.; Wimmer, H.; Staffen, W.; Hutzler, F.; Mair, A.; Ladurner, G. Developmental Dyslexia: Gray Matter Abnormalities in the Occipitotemporal Cortex. Hum. Brain Mapp. 2008, 29, 613–625.
- Siok, W.T.; Niu, Z.; Jin, Z.; Perfetti, C.A.; Tan, L.H. A Structural–Functional Basis for Dyslexia in the Cortex of Chinese Readers. Proc. Natl. Acad. Sci. USA 2008, 105, 5561–5566.
- Hu, W.; Lee, H.L.; Zhang, Q.; Liu, T.; Geng, L.B.; Seghier, M.L.; Shakeshaft, C.; Twomey, T.; Green, D.W.; Yang, Y.M.; et al. Developmental Dyslexia in Chinese and English Populations: Dissociating the Effect of Dyslexia from Language Differences. Brain 2010, 133, 1694–1706.
- Horwitz, B.; Rumsey, J.M.; Donohue, B.C. Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia. Proc. Natl. Acad. Sci. USA 1998, 95, 8939–8944.
- Stanberry, L.I.; Richards, T.L.; Berninger, V.W.; Nandy, R.R.; Aylward, E.H.; Maravilla, K.R.; Stock, P.S.; Cordes, D. Low-Frequency Signal Changes Reflect Differences in Functional Connectivity between Good Readers and Dyslexics during Continuous Phoneme Mapping. Magn. Reson. Imaging 2006, 24, 217–229.
- Shaywitz, S.E.; Shaywitz, B.A. Dyslexia (Specific Reading Disability). Biol. Psychiatry 2005, 57, 1301–1309.
- Martin, J.; Colé, P.; Leuwers, C.; Casalis, S.; Zorman, M.; Sprenger-Charolles, L. Reading in French-Speaking Adults with Dyslexia. Ann. Dyslexia 2010, 60, 238–264.
- Breznitz, Z.; Shaul, S.; Horowitz-Kraus, T.; Sela, I.; Nevat, M.; Karni, A. Enhanced Reading by Training with Imposed Time Constraint in Typical and Dyslexic Adults. Nat. Commun. 2013, 4, 1486.
- Cavalli, E.; Colé, P.; Brèthes, H.; Lefevre, E.; Lascombe, S.; Velay, J.-L. E-Book Reading Hinders Aspects of Long-Text Comprehension for Adults with Dyslexia. Ann. Dyslexia 2019, 69, 243–259.
- Landerl, K.; Wimmer, H. Development of Word Reading Fluency and Spelling in a Consistent Orthography: An 8-Year Follow-Up. J. Educ. Psychol. 2008, 100, 150–161.
- Paizi, D.; Zoccolotti, P.; Burani, C. Lexical Reading in Italian Developmental Dyslexic Readers. In Reading and Dyslexia in Different Orthographies; Psychology Press: Florence, KY, USA, 2010; pp. 199–216. ISBN 0-203-85846-8.
- Geredakis, A.; Karala, M.; Ziavra, N.; Toki, E. Preliminary Measurements of Voice Parameters Using Multi Dimensional Voice Program. World J. Res. Rev. 2017, 5, 6.
- National Reading Panel (US); National Institute of Child Health and Human Development (US). Teaching Children to Read: An Evidence-Based Assessment of the Scientific Research Literature on Reading and Its Implications for Reading Instruction: Reports of the Subgroups; National Institute of Child Health and Human Development: Washinngton, DC, USA, 2000.
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A.; Rose, E.; Lindamood, P.; Conway, T.; Garvan, C. Preventing Reading Failure in Young Children with Phonological Processing Disabilities: Group and Individual Responses to Instruction. J. Educ. Psychol. 1999, 91, 579.
- Torgesen, J.K.; Alexander, A.W.; Wagner, R.K.; Rashotte, C.A.; Voeller, K.K.; Conway, T. Intensive Remedial Instruction for Children with Severe Reading Disabilities: Immediate and Long-Term Outcomes from Two Instructional Approaches. J. Learn. Disabil. 2001, 34, 33–58.
- Simos, P.G.; Fletcher, J.M.; Bergman, E.; Breier, J.I.; Foorman, B.R.; Castillo, E.M.; Davis, R.N.; Fitzgerald, M.; Papanicolaou, A.C. Dyslexia-Specific Brain Activation Profile Becomes Normal Following Successful Remedial Training. Neurology 2002, 58, 1203–1213.
- Alexander, A.W.; Slinger-Constant, A.-M. Current Status of Treatments for Dyslexia: Critical Review. J. Child Neurol. 2004, 19, 744–758.
- Nelson, J.M.; Harwood, H. Learning Disabilities and Anxiety: A Meta-Analysis. J. Learn. Disabil. 2011, 44, 3–17.
- Riddick, B. Living with Dyslexia: The Social and Emotional Consequences of Specific Learning Difficulties/Disabilities; Routledge: London, UK, 2012; ISBN 0-203-43260-6.
- Livingston, E.M.; Siegel, L.S.; Ribary, U. Developmental Dyslexia: Emotional Impact and Consequences. Aust. J. Learn. Difficulties 2018, 23, 107–135.
- Karami, J.; Rezaee, F.; Nosrati, R.; Abasi, M.; Siahkamari, R. The Study of Self-Esteem of Dyslexic Children in Elementary School in Kermanshah. J. Pediatr. Nurs. 2019, 5, 33–40.
- Lithari, E. Fractured Academic Identities: Dyslexia, Secondary Education, Self-Esteem and School Experiences. Int. J. Incl. Educ. 2019, 23, 280–296.
- Rosalina, E. The Correlation between Self-Esteem and Student’s Reading Comprehension. Engl. Lang. Teach. Educ. J. 2019, 2, 70–78.
- Giovagnoli, S.; Mandolesi, L.; Magri, S.; Gualtieri, L.; Fabbri, D.; Tossani, E.; Benassi, M. Internalizing Symptoms in Developmental Dyslexia: A Comparison between Primary and Secondary School. Front. Psychol. 2020, 11, 461.
- Zuppardo, L.; Serrano, F.; Pirrone, C.; Rodriguez-Fuentes, A. More Than Words: Anxiety, Self-Esteem and Behavioral Problems in Children and Adolescents With Dyslexia. Learn. Disabil. Q. 2021.
- Leloup, G.; Anders, R.; Charlet, V.; Eula-Fantozzi, B.; Fossoud, C.; Cavalli, E. Improving Reading Skills in Children with Dyslexia: Efficacy Studies on a Newly Proposed Remedial Intervention—Repeated Reading with Vocal Music Masking (RVM). Ann. Dyslexia 2021, 71, 60–83.
- Breznitz, Z. Enhancing the Reading of Dyslexic Children by Reading Acceleration and Auditory Masking. J. Educ. Psychol. 1997, 89, 103.
- Tomatis, A. Pourquoi Mozart?: Essai; Fixot: Paris, France, 1991; ISBN 2-87645-107-7.
- Ganschow, L.; Lloyd-Jones, J.; Miles, T.R. Dyslexia and Musical Notation. Ann. Dyslexia 1994, 44, 185–202.
- Oglethorpe, S. Instrumental Music for Dyslexics: A Teaching Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0-470-77799-0.
- Vladikovic, J. Gifted Learners, Dyslexia, Music, and the Piano: Rude, Inattentive, Uncooperative, or Something Else? Arizona State University: Tempe, AZ, USA, 2013; ISBN 1-303-20624-2.
- Koelsch, S. Brain Correlates of Music-Evoked Emotions. Nat. Rev. Neurosci. 2014, 15, 170–180.
- Zatorre, R.J.; Chen, J.L.; Penhune, V.B. When the Brain Plays Music: Auditory–Motor Interactions in Music Perception and Production. Nat. Rev. Neurosci. 2007, 8, 547–558.
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical Training Shapes Structural Brain Development. J. Neurosci. 2009, 29, 3019–3025.
- Eren, B. Music and Dyslexia: The Therapeutic Use of Instrument (Piano) Training with a Child with Dyslexia (A Case Study). J. Educ. Pract. 2017, 8, 97–108.
- Hudson, K.N.; Ballou, H.M.; Willoughby, M.T. Improving Motor Competence Skills in Early Childhood Has Corollary Benefits for Executive Function and Numeracy Skills. Dev. Sci. 2021, 24, e13071.
- Saletta, M.; Gladfelter, A.; Vuolo, J.; Goffman, L. Interaction of Motor and Language Factors in the Development of Speech Production. In Routledge Handbook of Communication Disorders; Routledge: London, UK, 2015; pp. 381–392. ISBN 0-203-56924-5.
- Lyytinen, H.; Ahonen, T.; Eklund, K.; Guttorm, T.K.; Laakso, M.-L.; Leinonen, S.; Leppanen, P.H.; Lyytinen, P.; Poikkeus, A.-M.; Puolakanaho, A. Developmental Pathways of Children with and without Familial Risk for Dyslexia during the First Years of Life. Dev. Neuropsychol. 2001, 20, 535–554.
- Viholainen, H.; Ahonen, T.; Cantell, M.; Lyytinen, P.; Lyytinen, H. Development of Early Motor Skills and Language in Children at Risk for Familial Dyslexia. Dev. Med. Child Neurol. 2002, 44, 761–769.
- Getchell, N.; Pabreja, P.; Neeld, K.; Carrio, V. Comparing Children with and without Dyslexia on the Movement Assessment Battery for Children and the Test of Gross Motor Development. Percept. Mot. Skills 2007, 105, 207–214.
- Sibley, B.A.; Etnier, J.L. The Relationship between Physical Activity and Cognition in Children: A Meta-Analysis. Pediatr. Exerc. Sci. 2003, 15, 243–256.
- Nicolson, R.I.; Fawcett, A.J. Dyslexia Is More than a Phonological Disability. DYSLEXIA-CHICHESTER- 1995, 1, 19–36.
- Ben-Yehudah, G.; Fiez, J.A. Impact of Cerebellar Lesions on Reading and Phonological Processing. Ann. N. Y. Acad. Sci. 2008, 1145, 260–274.
- Reynolds, D.; Nicolson, R.I.; Hambly, H. Evaluation of an Exercise-Based Treatment for Children with Reading Difficulties. Dyslexia 2003, 9, 48–71.
- Reynolds, D.; Nicolson, R.I. Follow-up of an Exercise-Based Treatment for Children with Reading Difficulties. Dyslexia 2007, 13, 78–96.
- Snowling, M.J.; Hulme, C. A Critique of Claims from Reynolds, Nicolson & Hambly (2003) That DDAT Is an Effective Treatment for Children with Reading Difficulties-?Lies, Damned Lies and (Inappropriate) Statistics?? Dyslexia 2003, 9, 127–133.
- Rack, J.P.; Snowling, M.J.; Hulme, C.; Gibbs, S. No Evidence That an Exercise-Based Treatment Programme (DDAT) Has Specific Benefits for Children with Reading Difficulties. Dyslexia 2007, 13, 97–104.
- Denton, C.A. Physical Exercise and Movement-Based Interventions for Dyslexia. Pediatrics 1999, 1150, 27–31.
- Ben-Soussan, T.D.; Glicksohn, J.; Berkovich-Ohana, A. From Cerebellar Activation and Connectivity to Cognition: A Review of the Quadrato Motor Training. BioMed Res. Int. 2015, 2015, 954901.
- Levit-Binnun, N.; Golland, Y. Finding Behavioral and Network Indicators of Brain Vulnerability. Front. Hum. Neurosci. 2012, 6, 10.
- Levit-Binnun, N.; Davidovitch, M.; Golland, Y. Sensory and Motor Secondary Symptoms as Indicators of Brain Vulnerability. J. Neurodev. Disord. 2013, 5, 26.
- Courchesne, E.; Townsend, J.; Akshoomoff, N.A.; Saitoh, O.; Yeung-Courchesne, R.; Lincoln, A.J.; James, H.E.; Haas, R.H.; Schreibman, L.; Lau, L. Impairment in Shifting Attention in Autistic and Cerebellar Patients. Behav. Neurosci. 1994, 108, 848.
- De Fano, A.; Leshem, R.; Ben-Soussan, T.D. Creating an Internal Environment of Cognitive and Psycho-Emotional Well-Being through an External Movement-Based Environment: An Overview of Quadrato Motor Training. Int. J. Environ. Res. Public. Health 2019, 16, 2160.
- Palva, S.; Palva, J.M. New Vistas for α-Frequency Band Oscillations. Trends Neurosci. 2007, 30, 150–158.
- Kujala, J.; Pammer, K.; Cornelissen, P.; Roebroeck, A.; Formisano, E.; Salmelin, R. Phase Coupling in a Cerebro-Cerebellar Network at 8–13 Hz during Reading. Cereb. Cortex 2007, 17, 1476–1485.
- Palva, J.M.; Monto, S.; Kulashekhar, S.; Palva, S. Neuronal Synchrony Reveals Working Memory Networks and Predicts Individual Memory Capacity. Proc. Natl. Acad. Sci. USA 2010, 107, 7580–7585.
- Lu, B.; Pang, P.T.; Woo, N.H. The Yin and Yang of Neurotrophin Action. Nat. Rev. Neurosci. 2005, 6, 603–614.
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-Derived Neurotrophic Factor and the Development of Structural Neuronal Connectivity. Dev. Neurobiol. 2010, 70, 271–288.
- Mandel, R.J.; Gage, F.H.; Thal, L.J. Spatial Learning in Rats: Correlation with Cortical Choline Acetyltransferase and Improvement with NGF Following NBM Damage. Exp. Neurol. 1989, 104, 208–217.
- Angelucci, F.; De Bartolo, P.; Gelfo, F.; Foti, F.; Cutuli, D.; Bossù, P.; Caltagirone, C.; Petrosini, L. Increased Concentrations of Nerve Growth Factor and Brain-Derived Neurotrophic Factor in the Rat Cerebellum After Exposure to Environmental Enrichment. Cerebellum 2009, 8, 499–506.
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF Is Essential for Hippocampal Plasticity and Learning. J. Neurosci. 2009, 29, 10883–10889.
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204.
- AL-SHAWI, R.; Hafner, A.; Chun, S.; Raza, S.; Crutcher, K.; Thrasivoulou, C.; Simons, P.; Cowen, T. ProNGF, Sortilin, and Age-related Neurodegeneration. Ann. N. Y. Acad. Sci. 2007, 1119, 208–215.
- Cotman, C.W.; Berchtold, N.C. Exercise: A Behavioral Intervention to Enhance Brain Health and Plasticity. Trends Neurosci. 2002, 25, 295–301.
- Gatt, J.M.; Kuan, S.A.; Dobson-Stone, C.; Paul, R.H.; Joffe, R.T.; Kemp, A.H.; Gordon, E.; Schofield, P.R.; Williams, L.M. Association between BDNF Val66Met Polymorphism and Trait Depression Is Mediated via Resting EEG Alpha Band Activity. Biol. Psychol. 2008, 79, 275–284.
- Voss Plasticity of Brain Networks in a Randomized Intervention Trial of Exercise Training in Older Adults. Front. Aging Neurosci. 2010, 2, 32.
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wójcicki, T.R.; Mailey, E.L.; et al. Neurobiological Markers of Exercise-Related Brain Plasticity in Older Adults. Brain. Behav. Immun. 2013, 28, 90–99.
- Ernfors, P.; Lee, K.-F.; Kucera, J.; Jaenisch, R. Lack of Neurotrophin-3 Leads to Deficiencies in the Peripheral Nervous System and Loss of Limb Proprioceptive Afferents. Cell 1994, 77, 503–512.
- Schwartz, P.M.; Borghesani, P.R.; Levy, R.L.; Pomeroy, S.L.; Segal, R.A. Abnormal Cerebellar Development and Foliation in BDNF−/− Mice Reveals a Role for Neurotrophins in CNS Patterning. Neuron 1997, 19, 269–281.
- Holschneider, D.P.; Yang, J.; Guo, Y.; Maarek, J.-M.I. Reorganization of Functional Brain Maps after Exercise Training: Importance of Cerebellar–Thalamic–Cortical Pathway. Brain Res. 2007, 1184, 96–107.
- Foti, F.; Laricchiuta, D.; Cutuli, D.; De Bartolo, P.; Gelfo, F.; Angelucci, F.; Petrosini, L. Exposure to an Enriched Environment Accelerates Recovery from Cerebellar Lesion. Cerebellum 2011, 10, 104–119.
- Dietrich, A. Transient Hypofrontality as a Mechanism for the Psychological Effects of Exercise. Psychiatry Res. 2006, 145, 79–83.
- Fink, A.; Graif, B.; Neubauer, A.C. Brain Correlates Underlying Creative Thinking: EEG Alpha Activity in Professional vs. Novice Dancers. NeuroImage 2009, 46, 854–862.
- Crabbe, J.B.; Dishman, R.K. Brain Electrocortical Activity during and after Exercise: A Quantitative Synthesis. Psychophysiology 2004, 41, 563–574.
- Swinnen, S.P. Intermanual Coordination: From Behavioural Principles to Neural-Network Interactions. Nat. Rev. Neurosci. 2002, 3, 348–359.
- De Zeeuw, C.I.; Hoebeek, F.E.; Bosman, L.W.J.; Schonewille, M.; Witter, L.; Koekkoek, S.K. Spatiotemporal Firing Patterns in the Cerebellum. Nat. Rev. Neurosci. 2011, 12, 327–344.
- Carbon, M.; Kingsley, P.B.; Tang, C.; Bressman, S.; Eidelberg, D. Microstructural White Matter Changes in Primary Torsion Dystonia: White Matter Changes in Primary Dystonia. Mov. Disord. 2008, 23, 234–239.
- Carbon, M.; Argyelan, M.; Habeck, C.; Ghilardi, M.F.; Fitzpatrick, T.; Dhawan, V.; Pourfar, M.; Bressman, S.B.; Eidelberg, D. Increased Sensorimotor Network Activity in DYT1 Dystonia: A Functional Imaging Study. Brain 2010, 133, 690–700.
This entry is offline, you can click here to edit this entry!
