Diabetes is a metabolic disease characterized by a persistent over-normal level of blood glucose that causes impressive morbidity and mortality worldwide. Persistent hyperglycemia imposes damage on other organs, such as the eye, heart, kidney, and skin, as well as the nervous system, and is strongly correlated with a myriad of diabetes-related complications. Legumes, as an excellent source of protein, peptides, and phytochemicals, have played significant roles in human health throughout human history. Some legume-derived peptides with encouraging anti-diabetic potential have been gradually reported. Their hypoglycemic mechanisms have also been clarified at some classic diabetes treatment targets, such as the insulin receptor signaling pathway or other related pathways involved in the progress of diabetes, and key enzymes including α-amylase, α-glucosidase, and dipeptidyl peptidase-IV (DPP-4)
- legume
- type 2 diabetes
- bioactive peptide
1. Introduction
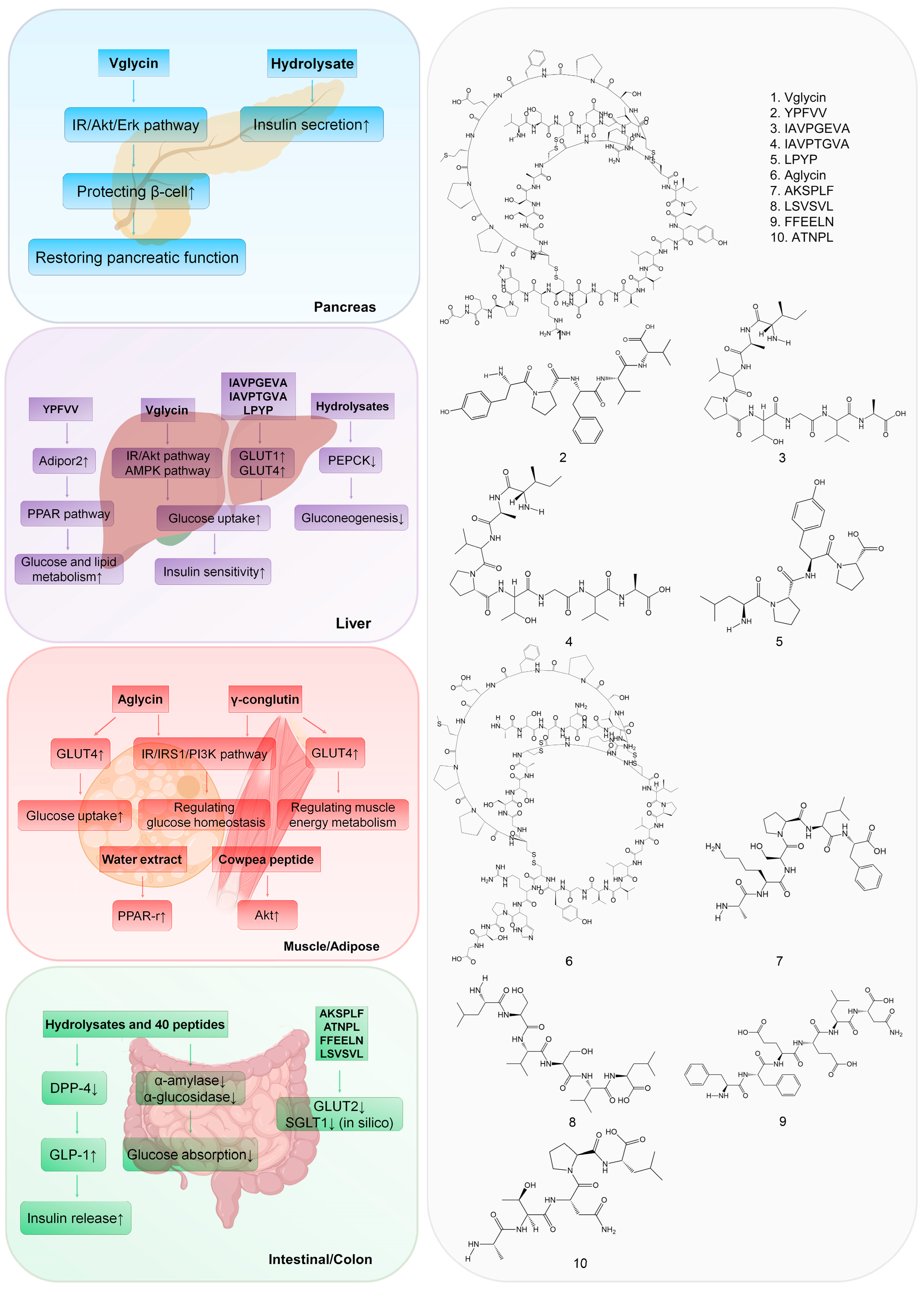
2. Targeting the Pancreas
3. Targeting the Liver
4. Targeting Muscle and Adipose Tissue
5. Targeting the Intestine and Colon
This entry is adapted from the peer-reviewed paper 10.3390/nu15051096
References
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107.
- Guo, S.D. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23.
- Jiang, H.; Feng, J.; Du, Z.; Zhen, H.; Lin, M.; Jia, S.; Li, T.; Huang, X.; Ostenson, C.G.; Chen, Z. Oral administration of soybean peptide Vglycin normalizes fasting glucose and restores impaired pancreatic function in Type 2 diabetic Wistar rats. J. Nutr. Biochem. 2014, 25, 954–963.
- Jiang, H.; Tong, Y.; Yan, D.; Jia, S.; Ostenson, C.G.; Chen, Z. The Soybean Peptide Vglycin Preserves the Diabetic beta-cells through Improvement of Proliferation and Inhibition of Apoptosis. Sci. Rep. 2015, 5, 15599.
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851.
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; González de Mejía, E. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159.
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition 2011, 27, 244–252.
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186.
- Yamada, Y.; Muraki, A.; Oie, M.; Kanegawa, N.; Oda, A.; Sawashi, Y.; Kaneko, K.; Yoshikawa, M.; Goto, T.; Takahashi, N.; et al. Soymorphin-5, a soy-derived mu-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARalpha systems in diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E433–E440.
- Yao, C.-C.; Tong, Y.-X.; Jiang, H.; Yang, D.-R.; Zhang, X.-J.; Zhang, P.; Su, L.; Zhao, Y.-Y.; Chen, Z.-W. Native polypeptide vglycin prevents nonalcoholic fatty liver disease in mice by activating the AMPK pathway. J. Funct. Foods 2020, 73, 104110.
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three Peptides from Soy Glycinin Modulate Glucose Metabolism in Human Hepatic HepG2 Cells. Int. J. Mol. Sci. 2015, 16, 27362–27370.
- Muñoz, E.B.; Luna-Vital, D.A.; Fornasini, M.; Baldeón, M.E.; Gonzalez de Mejia, E. Gamma-conglutin peptides from Andean lupin legume (Lupinus mutabilis Sweet) enhanced glucose uptake and reduced gluconeogenesis in vitro. J. Funct. Foods 2018, 45, 339–347.
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457.
- Barnes, M.J.; Uruakpa, F.O.; Udenigwe, C.C. Influence of Cowpea (Vigna unguiculata) Peptides on Insulin Resistance. J. Nutrit. Health Food Sci. 2015, 3, 1–3.
- Hilder, T.L.; Baer, L.A.; Fuller, P.M.; Fuller, C.A.; Grindeland, R.E.; Wade, C.E.; Graves, L.M. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J. Appl. Physiol. 2005, 99, 2181–2188.
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252.
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13.
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, A.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-mimetic action of conglutin-gamma, a lupin seed protein, in mouse myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205.
- Mojica, L.; Gonzalez de Mejia, E.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286.
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905.
- Ahren, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376.
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919.
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and alpha-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337.
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059.
- Ngoh, Y.Y.; Tye, G.J.; Gan, C.Y. The investigation of α-amylase inhibitory activity of selected Pinto bean peptides via preclinical study using AR42J cell. J. Funct. Foods 2017, 35, 641–647.
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005.
- Bompard-Gilles, C.; Rousseau, P.; Rougé, P.; Payan, F. Substrate mimicry in the active center of a mammalian α amylase: Structural analysis of an enzyme–inhibitor complex. Structure 1996, 4, 1441–1452.
- Nahoum, V.; Farisei, F.; Le-Berre-Anton, V.; Egloff, M.P.; Rouge, P.; Poerio, E.; Payan, F. A plant-seed inhibitor of two classes of alpha-amylases: X-ray analysis of Tenebrio molitor larvae alpha-amylase in complex with the bean Phaseolus vulgaris inhibitor. Acta Crystallogr. Sect. D Struct. Biol. 1999, 55, 360–362.
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410.
- Mojica, L.; de Mejia, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727.
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880.
- Arulmozhiraja, S.; Matsuo, N.; Ishitsubo, E.; Okazaki, S.; Shimano, H.; Tokiwa, H. Comparative Binding Analysis of Dipeptidyl Peptidase IV (DPP-4) with Antidiabetic Drugs—An Ab Initio Fragment Molecular Orbital Study. PLoS ONE 2016, 11, e0166275.
- Claessens, M.; Saris, W.H.; Van Baak, M.A. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br. J. Nutr. 2008, 100, 61–69.
