Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tissue Engineering (TE) is an interdisciplinary field that encompasses materials science in combination with biological and engineering sciences. Polymeric porous scaffolds play a critical role in TE strategies for providing a favorable environment for tissue restoration and establishing the interaction of the biomaterial with cells and inducing substances.
- tissue engineering
- biomaterials
- scaffolds
- regenerative medicine
1. Introduction
Human beings are made up of multiple complex tissues assembled in hierarchical structures that range from macro to nanoscale and fulfill specific roles to maintain the proper functioning of the body. These biological characteristics and structures inspired many scientists to design advanced multifunctional materials for the replacement of organs and tissues [1]. Every year, millions of patients suffer total or partial damage to their organs and tissues, and they are potential candidates for studies in the field of regenerative medicine. On the other hand, human life expectancy has quadrupled in the last three centuries, and the great drawback of conventional treatments lies mainly in the difficulty of finding donors and the rejection of the transplanted organ/tissue by the recipient organism [2][3]. This is how the field of tissue engineering (TE) in biomedicine was born, in order to develop functional tissues capable of regenerating and/or improving damaged tissue, and do so requires the contribution of several fields: TE, cell therapy, molecular therapy (e.g., gene and drug delivery), and artificial and bio-organ technology. TE started as a branch of regenerative medicine, and it is a rapidly growing research field in recent times. It combines engineering and biological science principles to create functional substitutes for native tissue and facilitate the maintenance, repair, and restoration of damaged tissue. In recent years, it has received considerable attention, as it is a promising field with a likely profound impact in field of medicine [4][5].
The term TE was officially created at a National Science Foundation workshop in 1988. TE consists of “the application of engineering and life science principles and methods toward the fundamental understanding of the structure-function relationship in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve tissue function” [6]. The roots of TE as a modern discipline lie in Boston, and the first recorded use of the term TE, as applied today, was published in an article titled “Functional Organ Replacement: The New Tissue Engineering Technology” in “Surgical Technology International” in 1991 [7]. Although the field of TE appears to be relatively new, the idea of replacing one tissue with another is as old as history itself. Examples are the Greek legend of “Prometheus” and the eternal regeneration of his liver, the miracle of the creation of Eve in “Genesis”, and the miraculous transplant of a member of the Holy Cosmos and Damian. With the introduction of the scientific method and advancements in our knowledge of traumatic injuries and diseases, the secrets of biology are better understood now [8].
TE and regenerative medicine are interdisciplinary fields that have evolved rapidly in recent years, and TE focuses on repairing and restoring the structural function of damaged tissue using various materials such as decellularized matrices, cells, scaffolds, and others [9]. A scaffold is a three-dimensional platform that can mimic the extracellular matrix (ECM) and is capable of providing mechanical, spatial, and biological signals to regulate and guide cellular responses [10]. Many researchers [11][12][13][14][15][16] use polymeric matrices composed of a mixture of one or more polymers (natural or synthetic) to solve the mechanical, thermal, and biological challenges that arise when they are used as scaffolds. Currently, the focus is on developing smart material devices that combine the benefits of different components, taking into account the specific biological, clinical, and medical aspects of the tissue defect.
2. Biomaterials for Tissue Engineering Scaffolds Fabrication
The definition of biomaterial emerged in 1976 at the First Consensus Conference of the European Society for Biomaterials (ESB). A biomaterial was defined as “a non-viable material used as a medical device, intended to interact with biological systems”. However, the current definition of biomaterial by ESB is “material intended to interact with biological systems to evaluate, treat, augment, or replace any tissue, organ, or function in the body” [6]. This subtle change in definition is indicative of how ESB has evolved in the field of biomaterials over time.
The repair and healing of the tissue usually involves the autograft technique as a conventional method, but it depends mainly on the availability of other donor tissue and on factors such as graft failure, pain, persistent bleeding, the risk of associated infectious diseases in the patients, etc. [17][18]. Current TE technologies have an advantage over traditional technology, since studies have focused on choosing materials with beneficial characteristics to build scaffolds that are highly compatible with the human body and its tissues. This is of great importance as it reduces the risk of immune rejection and the susceptibility to infection [19]. Biomaterials can be used as implants in the form of sutures, bone, joint replacements, ligaments, vascular grafts, heart valves, intraocular lenses, dental implants, and medical devices such as pacemakers, biosensors, etc. [20]. Therefore, bio-based materials are changing the dynamics of 21st century materials. The use of bio-based materials in various research areas is becoming more frequent, as they have potential applications in health care to improve the quality of life of many people [21].
An important concept in TE strategies is that of the scaffold, defined as a highly porous three-dimensional (3D) matrix capable of providing an adequate surface for cells adhesion and interacting with the biomolecules of interest (Figure 1). The composition and internal architecture of the scaffolds control cell behavior and well-being [6][22] by acting as a supporting prosthesis in vivo or as a cell adhesion substrate for TE in vitro [23]. The scaffolds shape the macroscopic level of the organs and tissues to be replaced without recreating the details that are observed at the nanoscale in real organs (Figure 2). However, the nanoarchitecture of the ECM provides an intricate fibrillar system in which specific molecular interactions occur between various ratios, isoforms, and geometric shapes of elastins, collagens, proteoglycans, and adhesion proteins such as laminins and fibronectins. This creates an environment with informational signals and instructions that guide cellular behavior to form complex tissues such as bone, liver, heart, and kidney tissue [24].
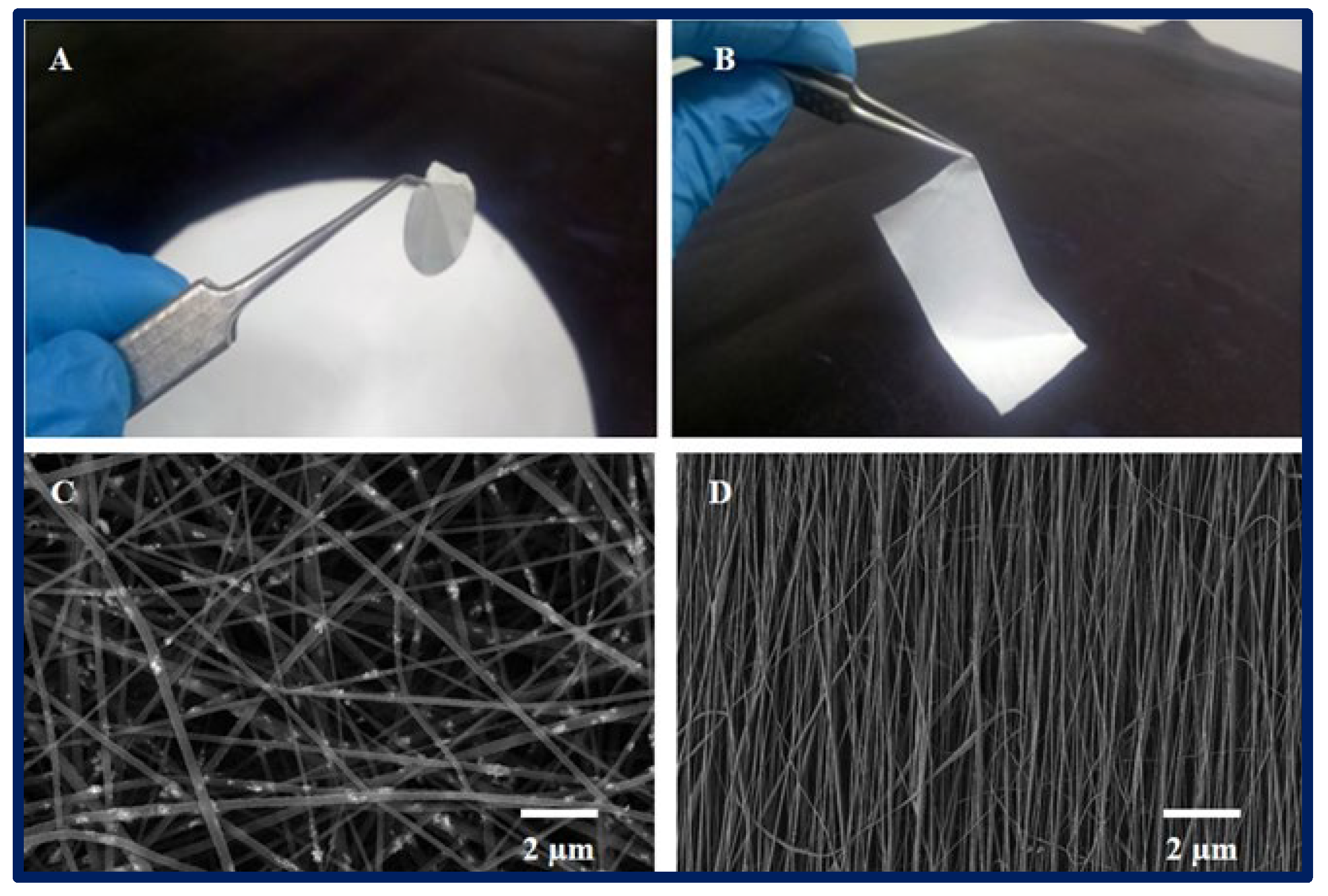
Figure 1. (A,B): macroscopic appearance of a 2D PCL scaffold fabricated by electrospinning technique. (C,D): microarchitecture of scaffolds observed under scanning electron microscopy, (C): randomly oriented nanofibers, and (D): nanofibers with parallel orientation.
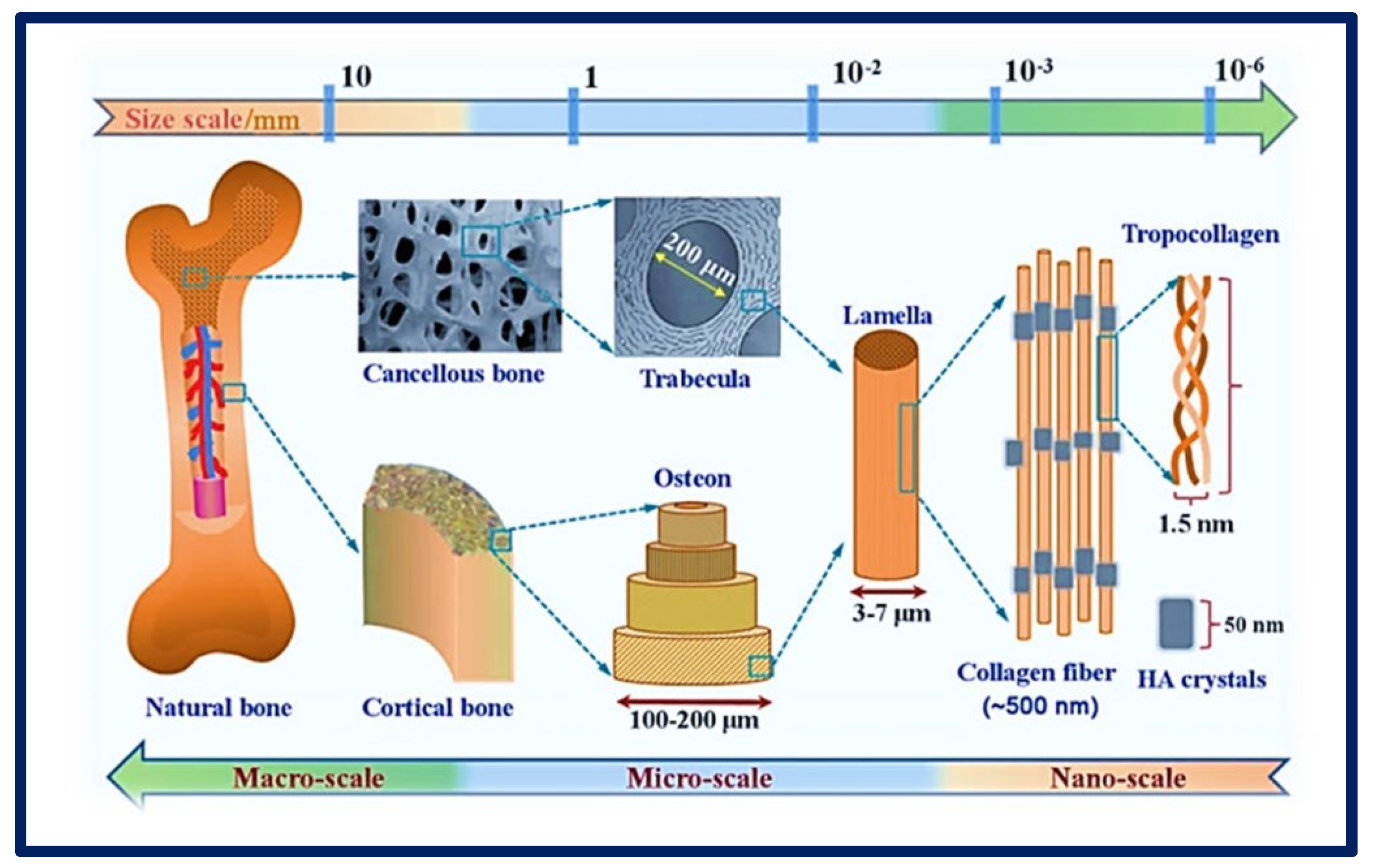
Figure 2. Complexity of an organ showing its macro, micro, and nanoarchitecture. Chemical composition and multi−scale structure of natural bone. Bibliography consulted [25] is licensed under CC BY 2.0.
Currently, various approaches can be applied to improve the biological and mechanical characteristics of scaffolds by combining design and manufacturing strategies with new materials. For the design of a good scaffold, it is of great importance to evaluate certain criteria before choosing a technique or its manufacture (Table 1). Parameters such as the surface characteristics (topography and roughness) and porosity (pore shape and size) of the scaffold must be controlled to increase cell migration into and on the surface of the scaffold [26][27] and to favor the efficient transport of metabolites without significantly compromising its mechanical stability [28]. The final applications of the scaffolds can vary significantly. They can be implanted empty (acellular) when they are expected to be colonized and invaded by host cells in a short time, or they may need to be previously seeded with appropriate cells before implantation or pre-cultured with cells in vitro in a suitable culture medium [29]. It is important to note that most mammalian cells depend on anchorage. Cell adhesion plays a crucial role in the development and maintenance of tissues, since it stimulates the signals that regulate the cell cycle, migration, differentiation, and cell survival. Therefore, it is important to design scaffold matrices that increase cell affinity to promote an ideal environment for cell attachment [30][31].
An ideal TE biomaterial not only mimics the native ECM of the tissue and provides mechanical support but also allows vascularization and integration with the host tissue, and gradually biodegrades and is remodeled with time as new tissues are formed. In this way, the native tissue can integrate with the scaffold and gradually replace the area originally occupied by it [32][33].
| Feature | Description |
|---|---|
| Adequate intrinsic physical and mechanical properties | This is defined by the microarchitecture and surface microtextures (surface topography). An ideal microarchitecture should be highly porous, with defined and interconnected pore sizes and a high surface area to volume ratio to allow for better vascularization, mass transfer, and cell growth. |
| Biocompatibility | It must produce the desired effect, be safe, and cause the minimum degree of inflammation once implanted. |
| Bioactivity | The biomaterial-cell interaction favors cell adhesion and proliferation, facilitating contact between cells and their migration over a prolonged period. Therefore, scaffolds can include biological molecules on their surface to promote cell adhesion or can also serve as a delivery vehicle or reservoir for growth-stimulating substances such as growth factors to accelerate regeneration. |
| Mimic EMC | It must be capable of mimicking the native tissue, providing an environment of optimal protection and nutrition. |
| Bioabsorption | It must be bioabsorbed in a controlled and appropriate time so that the new tissue replaces the space initially occupied by the biomaterials. |
| Versatility | They must be adaptable to different manufacturing techniques. |
| Translational perspective | The scaffold must be reproducible, accessible, and scalable to enable its use in high-demand applications for large tissues. |
3. Polymers for Tissue Engineering
When the tissue is damaged after an accident or illness, it is not always possible to fully recover or have access to a donor in a short time. This is where polymer-based scaffolds can play a vital role in improving the quality of life of the patient by restoring tissue and maintaining a suitable environment to produce faster healing [19]. Polymer engineering represents a growing area that is often tailored to specific needs in terms of the design and manufacturing for a given application [35]. Although there are various materials available to produce scaffolds (Figure 3), they are usually chosen for the design and construction of scaffolds due to their wide range of properties, availability, and low cost [36]. In addition, they stand out as a special class of materials due to their flexibility and versatility. They have been used for many types of biomedical devices, such as dental [37], orthopedic [38], and cardiovascular [39] implants.
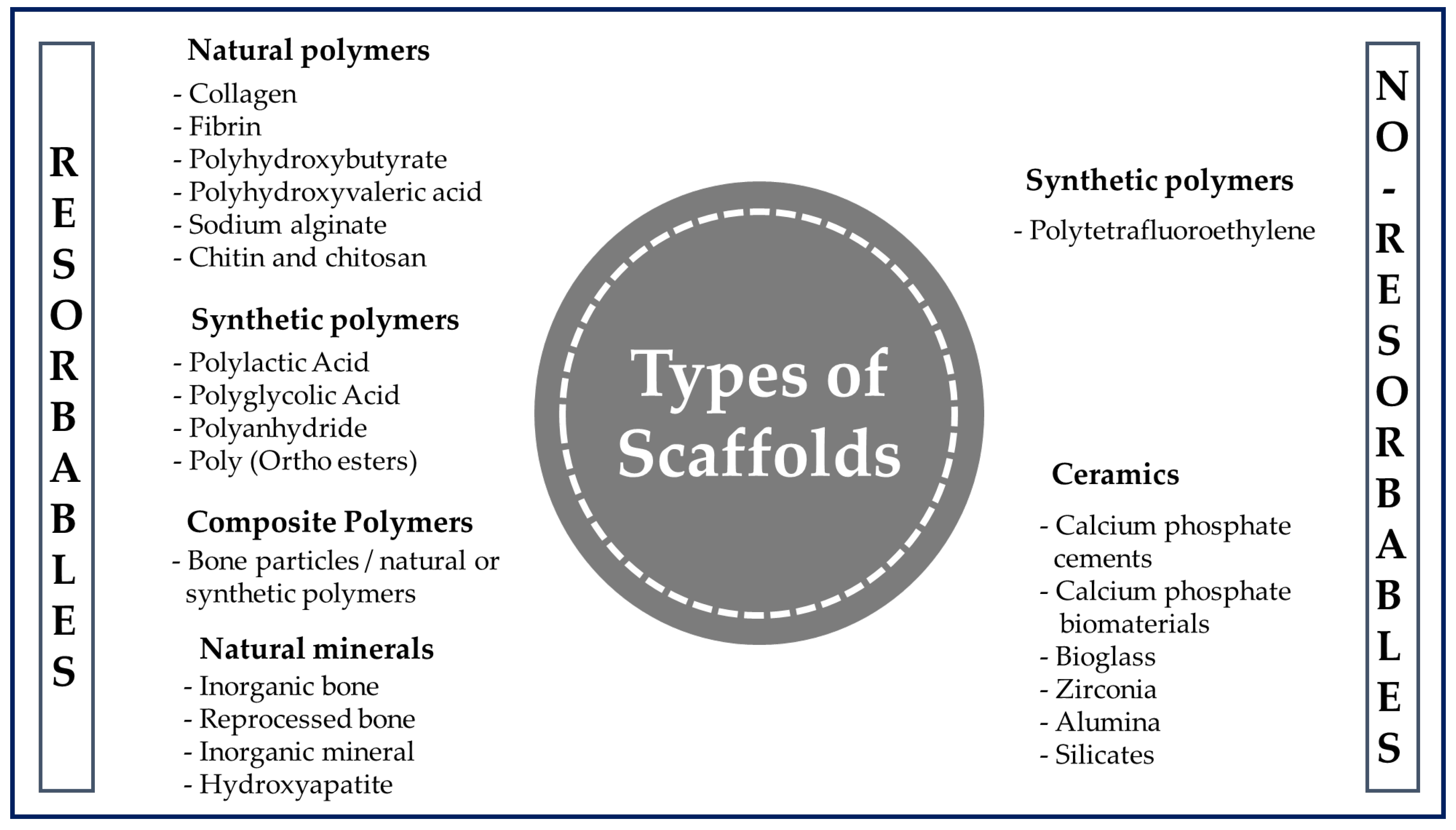
Figure 3. General classification of polymers for medical use.
A polymer is a long-chain macromolecule composed of repeating subunits called monomers that are connected by covalent bonds, and knowledge of the synthesis and manufacture of products from them is essential in the biomedical field [40]. Among the various classes of biomaterials available for medical uses (Figure 3), natural polymers (NP) and synthetic polymers (SP) are used the most often for the manufacture of scaffolds.
NPs are produced by biological systems and have been used in human applications such as pharmaceutical excipients, drug delivery, cosmetics, prostheses, and biomedical scaffolds [41]. NPs are components of the ECM, and these bioactive properties allow them to be recognized and degraded by the biological environment, increasing cell interaction with the biomaterial [42]. Also, they present less toxicity from chronic inflammation or immunological reactions than those observed with the use of SP [43] and can undergo chemical modifications and be potentially biodegradable and biocompatible [44]. For all of these reasons, as well as for their cost effectiveness and ready availability, the use of NPs is attractive in biomedical applications. Their disadvantages are their sensitivity to temperature increase, which causes these polymers to be destroyed before reaching their melting point; their complex structure, which makes them difficult to process; and the possibility of the transmission of diseases to humans from other species due to their sources of origin [42]. There are several examples of NPs that are used in clinical applications. Among the proteins are silk fibroin, collagen, gelatin, albumin, keratin, fibrinogen, elastin, and actin; the polysaccharides include chitosan, chitin, alginate, gellan gum, and derivatives; and the glycosaminoglycans include hyaluronic acid [20][43].
SPs appeared much later than NPs but have been playing crucial roles in recent times. They are more diverse and versatile for biomedical applications due to the ease of creating custom designs with them and making more controlled chemical modifications [43]. They also offer several further advantages over other materials used in developing TE scaffolds, such as the ability to tailor their mechanical properties and their more controlled degradation kinetics for various applications [45]. Often cheaper than biological scaffolds, they can be produced uniformly in large quantities and have a long storage time. Many commercially available SPs exhibit physical, chemical, and mechanical properties comparable to those of biological tissues [41]. The most widely used SPs approved by the FDA (Food and Drug Administration of the United States) are aliphatic, e.g., poly (lactic acid) (PLA), poly (glycolic acid) (PGA), and its copolymers poly (lactic-co-glycolic acid) (PLGA). They are linear aliphatic polyesters and degrade by the hydrolysis of their ester linkages, resulting in non-toxic products and metabolites that can be removed by the natural metabolism of the host [46]. Other linear aliphatic polyesters, such as poly (ε-caprolactone) (PCL) and poly (hydroxybutyrate) (PHB), are also used in TE research. PCL degrades at a significantly slower rate than PLA, PGA, and PLGA, making PCL less attractive for general TE applications, but more attractive for long-term implantation and the controlled release of substances [47]. Other SPs widely applied in TE to manufacture scaffolds are poly (propylene fumarate) (PPF) and poly (glycerol sebacate) (PGS). PPF is a good candidate for bone TE application as it is biocompatible, biodegradable, and osteoconductive and can be strengthened by cross-linking reactions [46]. Polymers are generally degraded by hydrolysis, producing intermediate natural metabolites, and have controllable degradation rates ranging from months to years. Therefore, scaffolds could be designed according to the requirement of each tissue by adjusting the initial proportion of the monomers. In addition, this is an advantage when polymers are required to release a molecule of interest, such as hormones and growth factors, in a controlled manner [48]. Despite all these advantages, many SPs tend to present an immune response or toxicity when combined with certain polymers that the host tissue cannot incorporate [43]. Therefore, one possible solution is to combine synthetic and natural polymers to overcome the deficiencies of each and obtain a scaffold that exploits the great merits of both [49].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10020218
References
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of bioinspired biomaterials for tissue regeneration. Adv. Health Mater. 2020, 27, e2000208.
- Abraham, G. Diseño y preparación de matrices poliméricas porosas para ingeniería de tejidos biológicos. Anales. Acad. Nac. CS Ex. Fis. Nat. 2017, 59, 115–130.
- Del Barrio Cortés, E.; Matutano Molina, C.; Rodríguez-Lorenzo, L.; Cubo-Mateo, N. Generation of Controlled Micrometric Fibers inside Printed Scaffolds Using Standard FDM 3D Printers. Polymers 2023, 15, 96.
- Ranjan, V.D.; Zeng, P.; Li, B.; Zhang, Y. In vitro cell culture in hollow microfibers with porous structures. Biomater. Sci. 2020, 8, 2175–2188.
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52.
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95.
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576.
- Vacanti, J.P.; Vacanti, C.A. The history and scope of tissue engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–8.
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Bio-medical Applications. Polymers 2021, 13, 3015.
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338.
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Al Ammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135.
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide–Polyaniline–Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845.
- Park, J.; Jung, J.-Y.; Shin, H.-W.; Park, J.-W.; Bang, J.; Huh, J. Loop and Bridge Conformations of ABA Triblock Comb Co-polymers: A Conformational Assessment for Molecular Composites. Polymers 2022, 14, 2301.
- Hansapaiboon, S.; Bulatao, B.P.; Sorasitthiyanukarn, F.N.; Jantaratana, P.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Fabrication of Curcumin Diethyl γ-Aminobutyrate-Loaded Chitosan-Coated Magnetic Nanocarriers for Improvement of Cytotoxicity against Breast Cancer Cells. Polymers 2022, 14, 5563.
- El-Dessouky, H.M.; McHugh, C. Multifunctional auxetic and honeycomb composites made of 3D woven carbon fibre preforms. Sci. Rep. 2022, 12, 22593.
- Castro, J.I.; Astudillo, S.; Mina Hernandez, J.H.; Saavedra, M.; Zapata, P.A.; Valencia-Llano, C.H.; Chaur, M.N.; Grande-Tovar, C.D. Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparti-cle/Orange Essential Oil Membranes for Biomedical Applications. Polymers 2023, 15, 135.
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527.
- Akbarzadeh, R.; Yousefi, A.-M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315.
- Ambekar, R.S.; Kandasubramanian, B. Progress in the advancement of porous biopolymer scaffold: Tissue engineering application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194.
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602.
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326.
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926.
- Chung, H.J.; Park, T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 249–262.
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138.
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059.
- Moreno, M.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Scaffolds for bone regeneration: State of the art. Curr. Pharm. Des. 2016, 22, 2726–2736.
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644.
- Sabino, M.A.; Loaiza, M.; Dernowsek, J.; Rezende, R.; Da Silva, J.V.L. Técnicas para la fabricación de andamios poliméricos con aplicaciones en ingenierÍa de tejidos. Rev. LatinAm. Metal. Mat. 2017, 37, 1–27.
- Perniconi, B.; Costa, A.; Aulino, P.; Teodori, L.; Adamo, S.; Coletti, D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 2011, 32, 7870–7882.
- Aguero Luztonó, L.; Zaldivar Silva, D.; Escobar Ivirico, J.L. Liberación de cefalexina a partir de hidrogeles de poli (acrilamida-co-ácido metacrílico). Biomecánica 2000, 18, 58–62.
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184.
- Chan, J.P.; Battiston, K.G.; Santerre, J.P. Synthesis and characterization of electrospun nanofibrous tissue engineering scaffolds generated from in situ polymerization of ionomeric polyurethane composites. Acta Biomater. 2019, 96, 161–174.
- Pierre, J.; Maria, K.; Solene-Emmanuelle, B.; Hany, N.; Valerie, V.; Mathieu, P.; Jerome, L.; Patricia, F.; Yong, C.; Philippe, M.; et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 2016, 80, 157–168.
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials 2009, 30, 4610–4617.
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620.
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 116–134.
- Yu, T.-T.; Cui, F.-Z.; Meng, Q.-Y.; Wang, J.; Wu, D.-C.; Zhang, J.; Kou, X.-X.; Yang, R.-L.; Liu, Y.; Zhang, Y.S.; et al. Influence of surface chemistry on adhesion and osteo/odontogenic differentiation of dental pulp stem cells. ACS Biomater. Sci. Eng. 2017, 3, 1119–1128.
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366.
- Venkatraman, S.; Boey, F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874.
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406.
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192.
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89.
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824.
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers—A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613.
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2018, 64, 1–36.
- Qian, Z.; Radke, D.; Jia, W.; Tahtinen, M.; Wang, G.; Zhao, F. Bioengineering scaffolds for regenerative engineering. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 444–461.
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40.
- Chaignaud, B.E.; Langer, R.; Vacanti, J.P. The history of tissue engineering using synthetic biodegradable polymer scaffolds and cells. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1996; pp. 1–14.
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109768.
This entry is offline, you can click here to edit this entry!
