Obesity has become a major metabolic disorder due to a combination of genetic, nutritional, and environmental factors. Energy balance in the body is sustained by regulating food intake and energy expenditure. Excessive calorie consumption and/or inadequate energy expenditure result in the accumulation of excess body fat, which eventually leads to an obese phenotype. Obesity has long been linked to increased susceptibility and severity of infectious diseases of the respiratory tract. Studies have shown that Body Mass Index (BMI) is linked to worse outcomes and increased severity of respiratory tract infections, such as non-allergic rhinitis and influenza-like illness. During the 2009 H1N1 pandemic and the ongoing SARS-CoV-2 (COVID-19) pandemic, obesity also became a significant risk factor for severe illness and higher mortality.
- COVID-19
- obesity
- innate and adaptive immunity
- inflammation
1. Introduction
2. Relationship between Obesity and Outcome of Infectious Diseases
2.1. Obesity and COVID-19
2.2. Obesity and H1N1 Infection
2.3. Obesity and Other Viral Respiratory Infections
3. Mechanism Linking Obesity to Increased Vulnerability to Infections
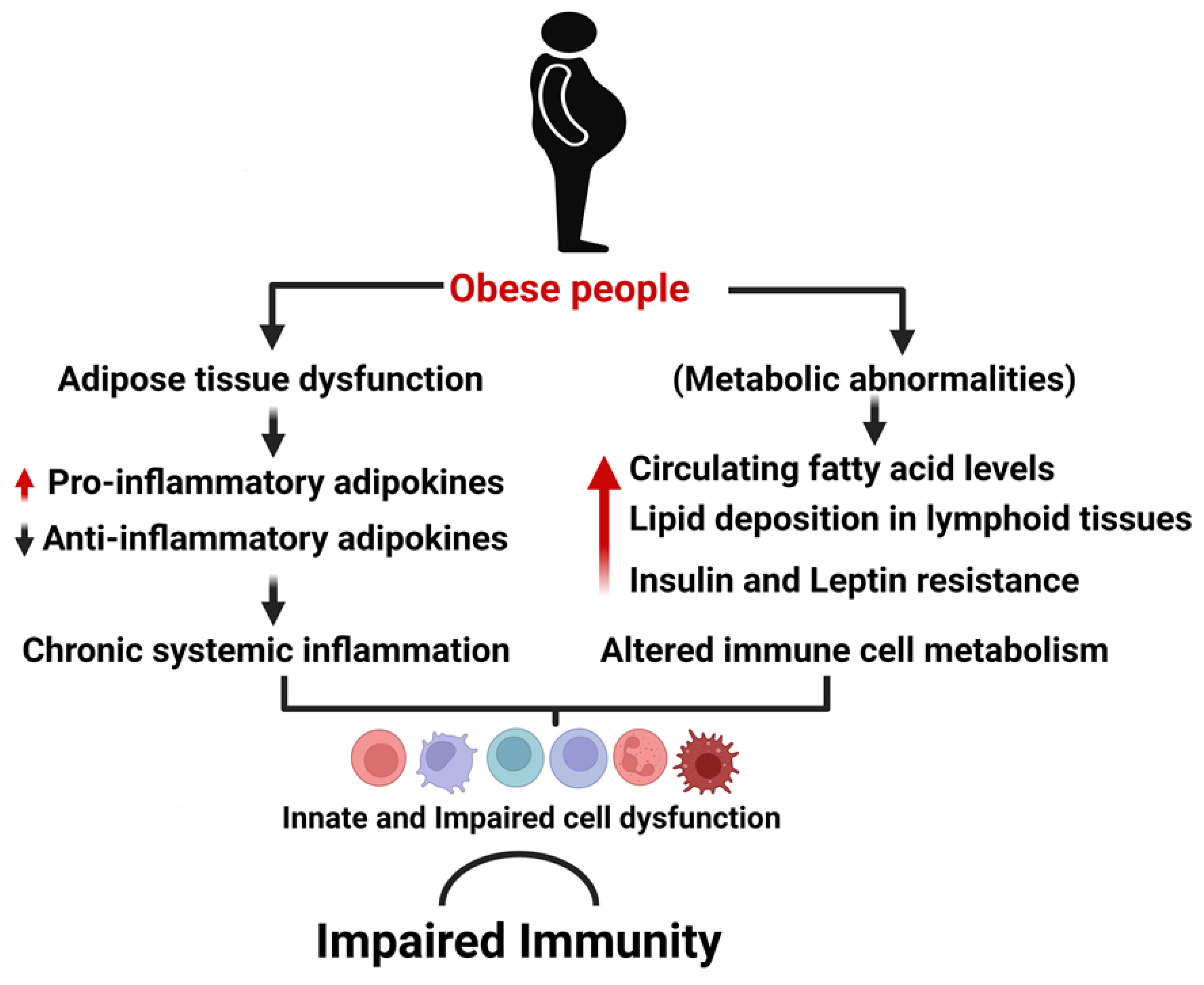
3.1. Obesity Associated Chronic Inflammation
3.2. Obesity and Immune System Integrity
3.3. Insulin and Leptin Resistance Impact Immune System Functioning
3.4. Pulmonary Complications of Obesity
This entry is adapted from the peer-reviewed paper 10.3390/obesities3010005
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9.
- Withrow, D.; Alter, D.A. The Economic Burden of Obesity Worldwide: A Systematic Review of the Direct Costs of Obesity. Obes. Rev. 2011, 12, 131–141.
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage On. Metabolism 2022, 133, 155217.
- Conway, B.; Rene, A. Obesity as a Disease: No Lightweight Matter. Obes. Rev. 2004, 5, 145–151.
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135.
- da Costa, L.A.; Arora, P.; García-Bailo, B.; Karmali, M.; El-Sohemy, A.; Badawi, A. The Association between Obesity, Cardiometabolic Disease Biomarkers, and Innate Immunity-Related Inflammation in Canadian Adults. Diabetes Metab. Syndr. Obes. 2012, 5, 347–355.
- Ghoorah, K.; Campbell, P.; Kent, A.; Maznyczka, A.; Kunadian, V. Obesity and Cardiovascular Outcomes: A Review. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 77–85.
- Black, P. Obesity and Diabetes: Time to Act. Br. J. Nurs. 2009, 18, 1089.
- Chan, M. Obesity and Diabetes: The Slow-Motion Disaster. Milbank Q. 2017, 95, 11–14.
- Shestakova, M.V.; Shestakova, E.A.; Sklyanik, I.A.; Stafeev, I.S. Obesity and Diabetes—Are They Always Together? Ter. Arkh. 2022, 94, 1131–1135.
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492.
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The Incidence of Co-Morbidities Related to Obesity and Overweight: A Systematic Review and Meta-Analysis. BMC Public Health 2009, 9, 88.
- Sharma, V.; Coleman, S.; Nixon, J.; Sharples, L.; Hamilton-Shield, J.; Rutter, H.; Bryant, M. A Systematic Review and Meta-analysis Estimating the Population Prevalence of Comorbidities in Children and Adolescents Aged 5 to 18 Years. Obes. Rev. 2019, 20, 1341–1349.
- Sullivan, P.W.; Morrato, E.H.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. Obesity, Inactivity, and the Prevalence of Diabetes and Diabetes-Related Cardiovascular Comorbidities in the U.S., 2000–2002. Diabetes Care 2005, 28, 1599–1603.
- He, Q.-X.; Zhao, L.; Tong, J.-S.; Liang, X.-Y.; Li, R.-N.; Zhang, P.; Liang, X.-H. The Impact of Obesity Epidemic on Type 2 Diabetes in Children and Adolescents: A Systematic Review and Meta-Analysis. Prim. Care Diabetes 2022, 16, 736–744.
- Yeh, T.-L.; Chen, H.-H.; Tsai, S.-Y.; Lin, C.-Y.; Liu, S.-J.; Chien, K.-L. The Relationship between Metabolically Healthy Obesity and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1228.
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood Obesity and Adult Cardiovascular Disease Risk Factors: A Systematic Review with Meta-Analysis. BMC Public Health 2017, 17, 683.
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined Effect of Obesity and Cardio-Metabolic Abnormality on the Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Cohort Studies. Int. J. Cardiol. 2013, 168, 4761–4768.
- Wang, Z.J.; Zhou, Y.J.; Galper, B.Z.; Gao, F.; Yeh, R.W.; Mauri, L. Association of Body Mass Index with Mortality and Cardiovascular Events for Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Heart 2015, 101, 1631–1638.
- Olsen, C.M.; Green, A.C.; Whiteman, D.C.; Sadeghi, S.; Kolahdooz, F.; Webb, P.M. Obesity and the Risk of Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2007, 43, 690–709.
- Sun, J.-W.; Zhao, L.-G.; Yang, Y.; Ma, X.; Wang, Y.-Y.; Xiang, Y.-B. Obesity and Risk of Bladder Cancer: A Dose-Response Meta-Analysis of 15 Cohort Studies. PLoS ONE 2015, 10, e0119313.
- Li, H.; Boakye, D.; Chen, X.; Hoffmeister, M.; Brenner, H. Association of Body Mass Index With Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, 2173–2183.
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk Factors for Endometrial Cancer: An Umbrella Review of the Literature. Int. J. Cancer 2019, 145, 1719–1730.
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5.
- Sohn, W.; Lee, H.W.; Lee, S.; Lim, J.H.; Lee, M.W.; Park, C.H.; Yoon, S.K. Obesity and the Risk of Primary Liver Cancer: A Systematic Review and Meta-Analysis. Clin. Mol. Hepatol. 2021, 27, 157–174.
- Global BMI Mortality Collaboration; di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-Mass Index and All-Cause Mortality: Individual-Participant-Data Meta-Analysis of 239 Prospective Studies in Four Continents. Lancet 2016, 388, 776–786.
- Huttunen, R.; Syrjänen, J. Obesity and the Risk and Outcome of Infection. Int. J. Obes. 2013, 37, 333–340.
- Mancuso, P. Obesity and Respiratory Infections: Does Excess Adiposity Weigh down Host Defense? Pulm. Pharmacol. Ther. 2013, 26, 412–419.
- Yuan, K.; Chen, H.-L. Obesity and Surgical Site Infections Risk in Orthopedics: A Meta-Analysis. Int. J. Surg. 2013, 11, 383–388.
- de Leeuw, A.J.M.; Oude Luttikhuis, M.A.M.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and Its Impact on COVID-19. J. Mol. Med. 2021, 99, 899–915.
- de Siqueira, J.V.V.; Almeida, L.G.; Zica, B.O.; Brum, I.B.; Barceló, A.; de Siqueira Galil, A.G. Impact of Obesity on Hospitalizations and Mortality, Due to COVID-19: A Systematic Review. Obes. Res. Clin. Pract. 2020, 14, 398–403.
- Mohammad, S.; Aziz, R.; al Mahri, S.; Malik, S.S.; Haji, E.; Khan, A.H.; Khatlani, T.S.; Bouchama, A. Obesity and COVID-19: What Makes Obese Host so Vulnerable? Immun. Ageing 2021, 18, 1.
- Yang, J.; Hu, J.; Zhu, C. Obesity Aggravates COVID-19: A Systematic Review and Meta-analysis. J. Med. Virol. 2021, 93, 257–261.
- Földi, M.; Farkas, N.; Kiss, S.; Zádori, N.; Váncsa, S.; Szakó, L.; Dembrovszky, F.; Solymár, M.; Bartalis, E.; Szakács, Z.; et al. Obesity Is a Risk Factor for Developing Critical Condition in COVID-19 Patients: A Systematic Review and Meta-Analysis. Obes. Rev. 2020, 21, e13095.
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T. A Novel Risk Factor for a Novel Virus: Obesity and 2009 Pandemic Influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312.
- Milner, J.J.; Rebeles, J.; Dhungana, S.; Stewart, D.A.; Sumner, S.C.J.; Meyers, M.H.; Mancuso, P.; Beck, M.A. Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice. J. Immunol. 2015, 194, 4846–4859.
- Wang, Y.; Hou, H.; Xu, J.; Wang, Y.; Yang, H. The Association between Obesity and ICU Admission among COVID-19 Patients: A Meta-Analysis of Adjusted Risk Estimates. Am. J. Emerg. Med. 2022, 56, 318–320.
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75.
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338.
- Fernández-Verdejo, R.; Moya-Osorio, J.L.; Fuentes-López, E.; Galgani, J.E. Metabolic Health and Its Association with Lifestyle Habits According to Nutritional Status in Chile: A Cross-Sectional Study from the National Health Survey 2016-2017. PLoS ONE 2020, 15, e0236451.
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645.
- Viardot, A.; Lord, R.V.; Samaras, K. The Effects of Weight Loss and Gastric Banding on the Innate and Adaptive Immune System in Type 2 Diabetes and Prediabetes. J. Clin. Endocrinol. Metab. 2010, 95, 2845–2850.
- Vasheghani, M.; Hessami, Z.; Rekabi, M.; Abedini, A.; Qanavati, A. Evaluating Possible Mechanisms Linking Obesity to COVID-19: A Narrative Review. Obes. Surg. 2022, 32, 1689–1700.
- Simón Abadía, C. COVID-19, a Graphic Account. Emergencias 2020, 32, 206–209.
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918.
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-Specific Mortality and Immunity Patterns of SARS-CoV-2. Nature 2021, 590, 140–145.
- Bergman, J.; Ballin, M.; Nordström, A.; Nordström, P. Risk Factors for COVID-19 Diagnosis, Hospitalization, and Subsequent All-Cause Mortality in Sweden: A Nationwide Study. Eur. J. Epidemiol. 2021, 36, 287–298.
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose Tissue: An Endocrine Organ Playing a Role in Metabolic Regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42.
- Dessie, Z.G.; Zewotir, T. Mortality-Related Risk Factors of COVID-19: A Systematic Review and Meta-Analysis of 42 Studies and 423,117 Patients. BMC Infect. Dis. 2021, 21, 855.
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345.
- Sawadogo, W.; Tsegaye, M.; Gizaw, A.; Adera, T. Overweight and Obesity as Risk Factors for COVID-19-Associated Hospitalisations and Death: Systematic Review and Meta-Analysis. BMJ Nutr. Prev. Health 2022, 5, 10–18.
- Singh, R.; Rathore, S.S.; Khan, H.; Karale, S.; Chawla, Y.; Iqbal, K.; Bhurwal, A.; Tekin, A.; Jain, N.; Mehra, I.; et al. Association of Obesity With COVID-19 Severity and Mortality: An Updated Systemic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2022, 13, 780872.
- Zhang, X.; Lewis, A.M.; Moley, J.R.; Brestoff, J.R. A Systematic Review and Meta-Analysis of Obesity and COVID-19 Outcomes. Sci. Rep. 2021, 11, 7193.
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population Risk Factors for Severe Disease and Mortality in COVID-19: A Global Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0247461.
- Chu, Y.; Yang, J.; Shi, J.; Zhang, P.; Wang, X. Obesity Is Associated with Increased Severity of Disease in COVID-19 Pneumonia: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2020, 25, 64.
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; el Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, Hypertension, Body Mass Index, Smoking and COVID-19-Related Mortality: A Systematic Review and Meta-Analysis of Observational Studies. BMJ Open 2021, 11, e052777.
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk Factors for Mortality in Patients with Coronavirus Disease 2019 (COVID-19) Infection: A Systematic Review and Meta-Analysis of Observational Studies. Aging Male 2020, 23, 1416–1424.
- Raeisi, T.; Mozaffari, H.; Sepehri, N.; Darand, M.; Razi, B.; Garousi, N.; Alizadeh, M.; Alizadeh, S. The Negative Impact of Obesity on the Occurrence and Prognosis of the 2019 Novel Coronavirus (COVID-19) Disease: A Systematic Review and Meta-Analysis. Eat. Weight. Disord. 2022, 27, 893–911.
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256.
- Yang, J.; Ma, Z.; Lei, Y. A Meta-Analysis of the Association between Obesity and COVID-19. Epidemiol. Infect. 2021, 149, e11.
- Zhao, X.; Gang, X.; He, G.; Li, Z.; Lv, Y.; Han, Q.; Wang, G. Obesity Increases the Severity and Mortality of Influenza and COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 595109.
- Fineberg, H.V. Pandemic Preparedness and Response—Lessons from the H1N1 Influenza of 2009. N. Engl. J. Med. 2014, 370, 1335–1342.
- Shah, N.K. H1N1 2009 Pandemic—Lessons Learnt. Indian Pediatr. 2011, 48, 363–364.
- Murphy, R.; Fragaszy, E.B.; Hayward, A.C.; Warren-Gash, C. Investigating Obesity as a Risk Factor for Influenza-like Illness during the 2009 H1N1 Influenza Pandemic Using the Health Survey for England. Influenza Other Respir. Viruses 2017, 11, 66–73.
- van Kerkhove, M.D.; Vandemaele, K.A.H.; Shinde, V.; Jaramillo-Gutierrez, G.; Koukounari, A.; Donnelly, C.A.; Carlino, L.O.; Owen, R.; Paterson, B.; Pelletier, L.; et al. Risk Factors for Severe Outcomes Following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011, 8, e1001053.
- Fezeu, L.; Julia, C.; Henegar, A.; Bitu, J.; Hu, F.B.; Grobbee, D.E.; Kengne, A.-P.; Hercberg, S.; Czernichow, S. Obesity Is Associated with Higher Risk of Intensive Care Unit Admission and Death in Influenza A (H1N1) Patients: A Systematic Review and Meta-Analysis. Obes. Rev. 2011, 12, 653–659.
- Díaz, E.; Rodríguez, A.; Martin-Loeches, I.; Lorente, L.; del Mar Martín, M.; Pozo, J.C.; Montejo, J.C.; Estella, A.; Arenzana, Á.; Rello, J. Impact of Obesity in Patients Infected With 2009 Influenza A(H1N1). Chest 2011, 139, 382–386.
- Kikukawa, T.; Ogura, T.; Harasawa, T.; Suzuki, H.; Nakano, M. H1N1 Influenza-Associated Pneumonia with Severe Obesity: Successful Management with Awake Veno-Venous Extracorporeal Membrane Oxygenation and Early Respiratory Physical Therapy. Acute Med. Surg. 2016, 3, 186–189.
- Tsatsanis, C.; Margioris, A.N.; Kontoyiannis, D.P. Association between H1N1 Infection Severity and Obesity—Adiponectin as a Potential Etiologic Factor. J. Infect. Dis. 2010, 202, 459–460.
- Kwong, J.C.; Campitelli, M.A.; Rosella, L.C. Obesity and Respiratory Hospitalizations during Influenza Seasons in Ontario, Canada: A Cohort Study. Clin. Infect. Dis. 2011, 53, 413–421.
- Rojas-Osornio, S.A.; Cruz-Hernández, T.R.; Drago-Serrano, M.E.; Campos-Rodríguez, R. Immunity to Influenza: Impact of Obesity. Obes. Res. Clin. Pract. 2019, 13, 419–429.
- Atamna, A.; Elis, A.; Gilady, E.; Gitter-Azulay, L.; Bishara, J. How Obesity Impacts Outcomes of Infectious Diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 585–591.
- Falagas, M.E.; Kompoti, M. Obesity and Infection. Lancet Infect. Dis. 2006, 6, 438–446.
- Karlsson, E.A.; Beck, M.A. The Burden of Obesity on Infectious Disease. Exp. Biol. Med. 2010, 235, 1412–1424.
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Duffaut, C.; Galitzky, J.; Lafontan, M.; Bouloumié, A. Unexpected Trafficking of Immune Cells within the Adipose Tissue during the Onset of Obesity. Biochem. Biophys. Res. Commun. 2009, 384, 482–485.
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587.
- Lu, J.; Zhao, J.; Meng, H.; Zhang, X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Front. Immunol. 2019, 10, 1173.
- Mraz, M.; Haluzik, M. The Role of Adipose Tissue Immune Cells in Obesity and Low-Grade Inflammation. J. Endocrinol. 2014, 222, R113–R127.
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose Tissue-Resident Immune Cells: Key Players in Immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415.
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132.
- Hotamisligil, G.S. Inflammation and Endoplasmic Reticulum Stress in Obesity and Diabetes. Int. J. Obes. 2008, 32, S52–S54.
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol. Cells 2014, 37, 365–371.
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, Chronic Inflammation, and Insulin Resistance. Cells 2022, 11, 3001.
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.A.; Meinders, A.E.; Jazet, I.M. Ectopic Fat and Insulin Resistance: Pathophysiology and Effect of Diet and Lifestyle Interventions. Int. J. Endocrinol. 2012, 2012, 983814.
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77.
- Adler, B.J.; Kaushansky, K.; Rubin, C.T. Obesity-Driven Disruption of Haematopoiesis and the Bone Marrow Niche. Nat. Rev. Endocrinol. 2014, 10, 737–748.
- van den Berg, S.M.; Seijkens, T.T.P.; Kusters, P.J.H.; Beckers, L.; den Toom, M.; Smeets, E.; Levels, J.; de Winther, M.P.J.; Lutgens, E. Diet-Induced Obesity in Mice Diminishes Hematopoietic Stem and Progenitor Cells in the Bone Marrow. FASEB J. 2016, 30, 1779–1788.
- Karlsson, E.A.; Sheridan, P.A.; Beck, M.A. Diet-Induced Obesity in Mice Reduces the Maintenance of Influenza-Specific CD8+ Memory T Cells. J. Nutr. 2010, 140, 1691–1697.
- Viardot, A.; Heilbronn, L.K.; Samocha-Bonet, D.; Mackay, F.; Campbell, L.V.; Samaras, K. Obesity Is Associated with Activated and Insulin Resistant Immune Cells. Diabetes Metab. Res. Rev. 2012, 28, 447–454.
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.A.; Dixit, V.D. Obesity Accelerates Thymic Aging. Blood 2009, 114, 3803–3812.
- Fischer, H.J.; Sie, C.; Schumann, E.; Witte, A.-K.; Dressel, R.; van den Brandt, J.; Reichardt, H.M. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J. Immunol. 2017, 198, 1910–1920.
- Helderman, J.H.; Strom, T.B. Specific Insulin Binding Site on T and B Lymphocytes as a Marker of Cell Activation. Nature 1978, 274, 62–63.
- Chi, H. Regulation and Function of MTOR Signalling in T Cell Fate Decisions. Nat. Rev. Immunol. 2012, 12, 325–338.
- Myers, D.R.; Wheeler, B.; Roose, J.P. MTOR and Other Effector Kinase Signals That Impact T Cell Function and Activity. Immunol. Rev. 2019, 291, 134–153.
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934.e4.
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises TCR Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. J. Immunol. 2010, 185, 1836–1845.
- Achari, A.; Jain, S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321.
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. Biomed. Res. Int. 2014, 2014, 658913.
- Jiang, Y.; Yi, C.; Yi, Y.; Jin, Q.; Kang, A.S.; Li, J.; Kumar Sacitharan, P. Adiponectin Exacerbates Influenza Infection in Elderly Individuals via IL-18. Signal Transduct. Target. Ther. 2020, 5, 32.
- Kearns, S.M.; Ahern, K.W.; Patrie, J.T.; Horton, W.B.; Harris, T.E.; Kadl, A. Reduced Adiponectin Levels in Patients with COVID-19 Acute Respiratory Failure: A Case-control Study. Physiol. Rep. 2021, 9, e14843.
- Flikweert, A.W.; Kobold, A.C.M.; van der Sar-van der Brugge, S.; Heeringa, P.; Rodenhuis-Zybert, I.A.; Bijzet, J.; Tami, A.; van der Gun, B.T.F.; Wold, K.I.; Huckriede, A.; et al. Circulating Adipokine Levels and COVID-19 Severity in Hospitalized Patients. Int. J. Obes. 2022, 47, 126–137.
- Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C.; Sánchez-Margalet, V. Role of Leptin in the Activation of Immune Cells. Mediat. Inflamm. 2010, 2010, 568343.
- Kim, S.Y.; Lim, J.H.; Choi, S.W.; Kim, M.; Kim, S.-T.; Kim, M.-S.; Cho, Y.S.; Chun, E.; Lee, K.-Y. Preferential Effects of Leptin on CD4 T Cells in Central and Peripheral Immune System Are Critically Linked to the Expression of Leptin Receptor. Biochem. Biophys. Res. Commun. 2010, 394, 562–568.
- Matarese, G. Leptin and the Immune System: How Nutritional Status Influences the Immune Response. Eur. Cytokine Netw. 2000, 11, 7–14.
- Procaccini, C.; la Rocca, C.; Carbone, F.; de Rosa, V.; Galgani, M.; Matarese, G. Leptin as Immune Mediator: Interaction between Neuroendocrine and Immune System. Dev. Comp. Immunol. 2017, 66, 120–129.
- de Candia, P.; Prattichizzo, F.; Garavelli, S.; Alviggi, C.; la Cava, A.; Matarese, G. The Pleiotropic Roles of Leptin in Metabolism, Immunity, and Cancer. J. Exp. Med. 2021, 218, e20191593.
- Ozata, M.; Ozdemir, I.C.; Licinio, J. Human Leptin Deficiency Caused by a Missense Mutation: Multiple Endocrine Defects, Decreased Sympathetic Tone, and Immune System Dysfunction Indicate New Targets for Leptin Action, Greater Central than Peripheral Resistance to the Effects of Leptin, and Spontaneous Correction of Leptin-Mediated Defects. J. Clin. Endocrinol. Metab. 1999, 84, 3686–3695.
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Cowley, M.A. Leptin Resistance and Obesity. Obesity 2006, 14, 254S–258S.
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163.
- Zhou, Y.; Rui, L. Leptin Signaling and Leptin Resistance. Front. Med. 2013, 7, 207–222.
- Dixon, A.E.; Peters, U. The Effect of Obesity on Lung Function. Expert. Rev. Respir. Med. 2018, 12, 755–767.
- Salome, C.M.; King, G.G.; Berend, N. Physiology of Obesity and Effects on Lung Function. J. Appl. Physiol. 2010, 108, 206–211.
- De Sant’Anna, M., Jr.; Carvalhal, R.F.; de Oliveira, F.d.F.B.; Zin, W.A.; Lopes, A.J.; Lugon, J.R.; Guimarães, F.S. Mecânica Respiratória de Pacientes Com Obesidade Mórbida. J. Bras. Pneumol. 2019, 45, e20180311.
- Bui, D.S.; Cassim, R.; Russell, M.A.; Doherty, A.; Lowe, A.J.; Agusti, A.; Dharmage, S.C.; Lodge, C.J. Lung Function Levels Influence the Association between Obesity and Risk of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 1106–1108.
- Caci, G.; Albini, A.; Malerba, M.; Noonan, D.M.; Pochetti, P.; Polosa, R. COVID-19 and Obesity: Dangerous Liaisons. J. Clin. Med. 2020, 9, 2511.
- Littleton, S.W.; Tulaimat, A. The Effects of Obesity on Lung Volumes and Oxygenation. Respir. Med. 2017, 124, 15–20.
- Melo, L.C.; da Silva, M.A.M.; do Nascimento Calles, A.C. Obesity and Lung Function: A Systematic Review. Einstein 2014, 12, 120–125.
- Soyak Aytekin, E.; Sahiner, U.M.; Tuten Dal, S.; Unsal, H.; Hakverdi, O.; Oguz, B.; Ozsurekci, Y.; Sekerel, B.E.; Soyer, O. Obesity Is a Risk Factor for Decrease in Lung Function after COVID-19 Infection in Children with Asthma. Pediatr. Pulmonol. 2022, 57, 1668–1676.
