Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Bioplastics are one of the possible alternative solutions to the polymers of petrochemical origins. Bioplastics have several advantages over traditional plastics in terms of low carbon footprint, energy efficiency, biodegradability and versatility.
- Bioplastics
- Biopolymers
- Conventional polymers
- Biodegradability
- Sustainability
- Renewable resources
1. Introduction
The use of polymeric materials is widely spread around the world. These materials have significant advantages compared with other, more conventional materials, such as metals and wood, mainly because of their properties and performance.
It is estimated that 99% of these polymeric materials come from fossil fuels. These plastics entail several issues since their primary raw material is a hazard to environment conservation [1].
The durability and degradability of these materials are two contradictory topics. For most applications, it is favourable that the material maintains specific properties throughout time, but it is also desirable to discard them easily after their use. There are some alternative processes usually used to manage this kind of waste: recycling (one of the most sustainable waste management processes but requires a controlled process to have a final product with good properties) and energy recovery (allows the production of energy by burning the waste but ends up producing toxic emissions and greenhouse gases) [2,3]. However, a massive quantity of material ends up in landfills or even abandoned, and some of it reaches the ocean. A long-term study took place on the North Atlantic Sea, where it was observed a seawater sample contained 580,000 pieces of plastic per square kilometre. This waste management has created a crisis, since landfills have a limited capacity, high costs and strict legislation [4].
The remaining percentage of plastics is produced from natural raw materials and are denominated bio-based plastics or bioplastics [1]. The use of bioplastics dates centuries ago. In 1500 BCE, Mesoamerican cultures (Maya, Aztecs) used natural rubber and latex to make containers and waterproof their clothes. However, only in 1862 was the first manmade bioplastic produced (Parkesine, a bioplastic made from cellulose), created by Alexander Parkes. The first company to produce bioplastics was Marlborough Biopolymers in 1983. They produced strips, filaments, chips, panels and powders of bacteria called Biopol. More recently, in 2018, Project Effective was launched with the goal of replacing nylon with bio-nylon, and it created the first bioplastic made from the fruit [5].
Although investigations regarding bioplastics have been done for over a century, their implementation and extensive production is not yet developed. Figure 1 presents a chart of the last few years’ production and the forecast for the years to come. In 2019, 1.95 Mt of bioplastic was produced, corresponding to about 0.6% of all plastic production worldwide.
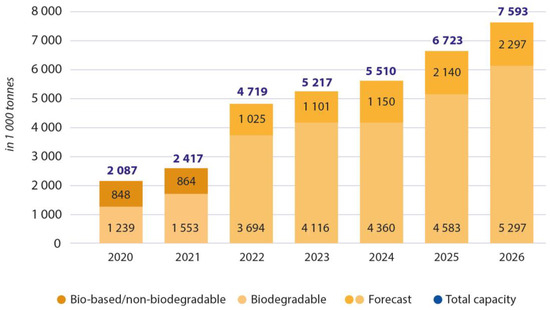
Figure 1. Global production capacities of bioplastics 2021–2026. Adapted from European Bioplastics, “Bioplastics Market Development Update 2021”. https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf (accessed on 29 December 2022) [6].
The small production of these plastics is mainly due to their more expensive manufacturing and generally inferior mechanical properties compared to fossil-based polymers. However, it is necessary to develop these materials to have a sustainable alternative to petrochemical materials [7]. This paper will discuss current scenarios and the inherent production limitations and present the pros and cons of producing and using bioplastics to replace some petrochemical-based polymers.
2. Bioplastics Composites
The synthetic assembly of two or more materials, a matrix binder and selected reinforcing agents, is used for various applications. The goal is to overcome the weaknesses and increase versatility. The most common fibres exercised in recent times are glass fibres, carbon fibres, aramid fibres, natural fibres, nylon and polyester fibres. There is a clear advantage to using natural-based fibres; for example, they are biodegradable, renewable, available in bulk, cheaper and lighter [54,55,56].
2.1. Coating
Coating bioplastics is an excellent technique to improve some of the properties of these materials; it is specially used to enhance the barrier properties. By applying a thin layer of other polymers on top of the bioplastic, the tensile strength and elasticity can be improved, as well as increasing oxygen and water vapour permeability and resistance. Some examples of usually used coatings are listed below:
-
PLA barrier properties (oxygen and water vapour) can be improved by applying a PLA-Si/SiOx, AlOx (aluminium oxide), PCL-Si/SiOx or PEO-Si/SiOx (polyethylene oxide) coating.
-
When coated with PLA, SPI (soy protein isolate) films tensile strength increases from 2.8 to 17.4 MPa, and the elongation went from 165.7% to 203.4%. However, the water vapor permeability decreases 20- to 60-fold, depending on the PLA concentration in the coating solution.
-
Nitrocellulose or PVdC (polyvinylidene chloride) coating on cellophane is also used to improve oxygen and water vapour barrier properties.
-
Coating acetylated cellulose film with PHB increases elastic modulus and tensile strength for films containing 10% or more PHB and a better strain at break for films containing 15% or more PHB while lowering the water vapour permeability values [8].
2.2. Nanocomposites
For a composite to be considered a nanocomposite, it must have at least one of its types of particles with dimensions in the nano range. The composite can be classified as polymer layered crystal nanocomposites (Figure 7a), nanotubes or whiskers (Figure 2b) and isodimensional nanoparticles (Figure 7c), according to the number of dimensions it has: three, two or one, respectively [2].
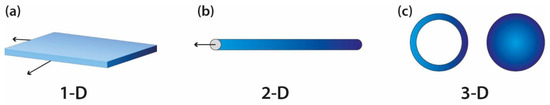
Figure 2. Nanoparticle geometries: (a) layered particles (1D), (b) acicular or fibrous ones (2D) and (c) isodimensional nanoparticles (3D). Adapted from “Block copolymer nanocomposites” [57].
The use of these materials as reinforcement to other polymers depends on the capacity of the matrix (continuous phase) to interact with the fibre (discontinuous phase). There are several ways to mix the phases of the composite; one of them is situ polymerization, which involves the dissolution of the nanoparticles in the monomer solution before polymerization; the addition of the nanoparticles during the extrusion process, a process called melt intercalation; or solvent intercalation (use of a solvent to enhance the affinity between the nanoparticles and the matrix). These processes help change some of the composite properties according to the intention.
Nanoclays are one of the most used fibres as reinforcement of bioplastics. They are usually used as layered particles of 1 µm. Different affinities between the matrix and the nanofibers create different interactions: tactoid, intercalated and exfoliated. The first case occurs when the interaction between the continuous and discontinuous phases is low; this happens because the clay interlayer does not expand within the matrix, so no true nanocomposite is formed. When the affinity is moderate, it is possible for a part of the polymer to penetrate the clay interlayer, since there was some level of expansion of the fibre; this creates an intercalated structure of matrix and fibre. The last situation entails a high affinity, the clay disperses into the polymeric matrix, and the layered structure is lost, forming an exfoliated structure instead [8] (Figure 3).
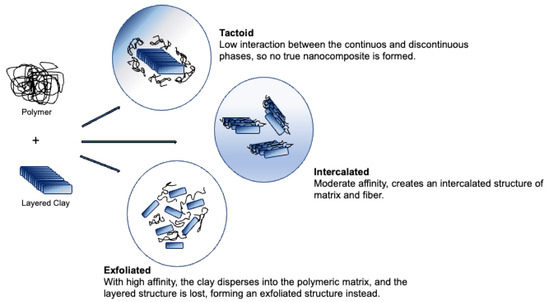
Figure 3. Structures of polymer nanoclay composite.
It is also possible to create composites using bigger particles as reinforcement. The project EcoPlast works with melt intercalation using natural fibres (sawdust, cellulose and cork) and biodegradable polymers as matrices (such as PLA, PBS (polybutylene succinate), starch, cellulose and PCL) in several different combinations. The materials used are represented in Figure 4. They are within different ranges of dimensions and densities, where (a) (less than 0.7 mm) and (b) (between 0.7 and 1.4 mm) are quite homogeneous. At the same time, (c) (between 1.4 and 2.8 mm) has very different particles in shape, size and colouring.

Figure 4. Different batches of sawdust used (a) (less than 0.7 mm), (b) (between 0.7 and 1.4 mm) and (c) (between 1.4 and 2.8 mm). From the project EcoPlast.
The dispersion of the fibres is affected by the hydrophobic/hydrophilic character of the polymer and the clay; this can be adapted with chemical modifications such as cationic exchange, ionomers, block copolymers adsorption and organosilane grafting. Since a high surface-to-volume ratio leads to better polymer properties, the exfoliated structure is preferred.
Some of the properties affected are the elongation at break (especially when using PLA film as matrix); barrier properties, explained by the confinement effect (the molecules of the polymeric matrix penetrate the dispersed nanoparticles, creating a denser material and creating a more tortuous path for the water and gas molecules to travel through) and thermal stability [8].
2.3. Cellulose
Adding cellulose to the bioplastic is another way to influence some properties of the final material. It is possible to have good adhesion between the fibre and the matrix due to the chemical similarity between starch and natural fibres. The main effect this addition has on the composite is the reduction of water vapour permeability due to the fibres’ highly crystalline and hydrophobic character, also affecting the Young’s modulus, tensile strength and the elongation break [8].
This entry is adapted from the peer-reviewed paper 10.3390/polym15030517
This entry is offline, you can click here to edit this entry!
