Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cell sheet engineering is an advanced scaffold-free tissue engineering technique applicable to repairing or regenerating defective tissues and organs. It has proven to be an important breakthrough technology in regenerative medicine.
- cell sheet technique
- tissue engineering
- regenerative medicine
- temperature-responsive polymer
- transplantation
1. Introduction
Regenerative medicine aims to replace or regenerate dysfunctional cells, tissues, or organs to restore them to their original function. This is a promising research field for diseases that have, so far, been incurable. Conventional regenerative therapies, including cell suspensions, injections, scaffold-embedded cells, and other tissue engineering methods, are among the advanced approaches in regenerative medicine; however, cell loss from injection sites [1] and the lack of cell-to-cell and extracellular matrix (ECM) interactions, which significantly contribute to the properties and function of each organ and tissue, are the main challenges the techniques face [2]. This negatively affects the ability to provide signals to the cell population and promote cell adhesion, survival, and proliferation, resulting in low repair efficiency after tissue grafting [3].
Cell sheet engineering is an advanced scaffold-free tissue engineering technique applicable to repairing or regenerating defective tissues and organs, including the heart [2][4], skin [5], cornea [6], cartilage [7], esophagus [8], and brain [9]. It was first developed and published in 1990 by Yamada et al. [10]. To engineer cell sheets, the desired cells should be grown to confluence on the culture surface coated with a temperature-responsive polymer such as poly(N-isopropylacrylamide) (PIPAAm), which allows intact cells to be harvested without enzymatic treatment. As a result, cells are formed as monolayers along with their deposited ECM [11], intact cell surface proteins, and receptors, which play vital roles in the functional tissue. Furthermore, cell sheets can be transplanted directly into the target tissue or even used to create three-dimensional (3D) tissue-like structures [12][13]. This approach exhibits numerous advantages over conventional regenerative therapies, such as cell injection and tissue reconstruction with biodegradable scaffolds [14]. To date, cell sheet engineering has been utilized for many different applications in vitro and in vivo, as well as in clinical trials.
In addition, other new cell-based sheet fabrication techniques have been developed using multidisciplinary technologies to fabricate thicker, more functional, complex, and homogeneous tissues.
2. Techniques for Harvesting Cell Sheets
One of the most important steps in generating scaffold-free tissue is harvesting intact cells from the culture surface. Platforms should be designed to allow cells to adhere and detach without digesting the ECM, which acts as a glue between cell layers. Additionally, the platform should preserve all signaling proteins and molecules important for promoting cellular functions and biological processes. Various systems have been designed to harvest cell sheets without treatment with proteases such as trypsin. To date, several technologies have been explored to harvest cell sheets. These include temperature-responsive systems using synthetic polymers and non-temperature-responsive systems, such as the ion-induced cell-detachment method, electro-responsive systems, photo-responsive systems, pH-responsive systems, mechanical systems, and magnetic systems. Examples of cell sheet fabrication methods using different platforms are presented in Table 1.
Table 1. Cell sheet harvesting system and detachment time.
| Cell Types | Responsive System on TCPS | Detachment Temperature/Time | Refs. |
|---|---|---|---|
| BAECs | PIPAAm on TCPS | 20 °C/~75 min | [15][16] |
| PIPAAm/microporous membrane | 20 °C/~30 min | [15] | |
| PIPAAm/PEG/microporous membrane | 20 °C/~19 min | [17] | |
| A comb-type grafted PIPAAm | 20 °C/~25 min | [18] | |
| PIPAAm/PHEMA | 20 °C/~30 min | [19] | |
| PIPAAm/PAAm Poly(IAAm-co-CIPAAm) |
20 °C/~30 min 20 °C/~35 min |
[20] [21] |
|
| Dermal fibroblast | MC/PBS/Col (8% MC, PBS MW = 77,000–94,000, 10 g/L PBS) |
20 °C/~10–20 min | [22] |
| Human-adipose-tissue-derived stem cells | MC/PBS/Col (12% to 16% MC, MW = 15,000, 1.5 M PBS) |
RT (~30 °C)/~2–3 min | [23] |
| Dermal fibroblast, MSC, myoblasts, endothelial cells | DVB/4VP/Ion-induction | 37 °C is possible/~100 s | [24] |
| Dermal fibroblasts | Electrical responsive system | 37 °C is possible/~5 min | [25] |
2.1. Temperature-Responsive Systems Using PIPAAm
Several synthetic polymers with molecular architectures are responsive to changes in pH, electric field, chemical species, and temperature, among other environmental factors. PIPAAm is a material of great interest and is widely used in biomedical fields owing to its unique behavior: reversible solubility in an aqueous solution with a change in temperature, as first reported by Heskins and Guillet [26]. Polymer chains of PIPAAm hydrate to form an expanded structure in water at temperatures below its lower critical solution temperature (LCST) of 32 °C. However, these chains form a compact structure upon dehydration at temperatures higher than the LCST. This transition of the aqueous PIPAAm solution is mainly due to conformational changes in the polymer chain arising from hydration changes in the isopropyl side groups [27].
To harvest cell sheets, Okano’s research group pioneered the development of temperature-responsive culture plates by grafting a PIPAAm layer on a tissue culture polystyrene surface (TCPS) through the electron beam polymerization method [10][28] (Figure 1A). At 37 °C, the culture surface is hydrophobic, and cells are allowed to attach and proliferate. By decreasing the temperature to 20 °C, the culture surface becomes hydrophilic, and the cells easily detach from the surface [10][29][30] (Figure 1B). However, detachment of cell sheets from surfaces of TCPS grafted with PIPAAm is slow (~75 min to detach primary bovine aortic endothelial cells (BAECs)), occurring gradually from the periphery of the sheet toward the interior. Thus, a longer time is required to detach an intact cell sheet completely. In principle, the limiting step in detaching cell sheets is the diffusion of water molecules beneath the PIPAAm-grafted surface. Therefore, approaches have been considered to accelerate the hydration of hydrophobic PIPAAm chains. These include the introduction of a highly water-permeable microporous membrane between the cell sheet and the PIPAAm surface, reducing the detachment time of BAECs to within 30 min [15]. Other approaches to accelerate cell sheet detachment include grafting PIPAAm with poly(ethylene glycol) (PEG) onto porous cell culture membranes (from which BAECs take 19 min to detach) [17], developing comb-type grafted PIPAAm gels on a TCPS (taking 25 min for BAECs to detach) [18] and grafting poly(2-hydroxyethyl methacrylate) (PHEMA) and PIPAAm onto TCPS (from which BAECs take 30 min to detach) [19]. Furthermore, modification of the PIPAAm culture surface for rapid cell sheet harvesting has been demonstrated by grafting an ultrathin double polymeric nanolayer consisting of PIPAAm and hydrophilic polyacrylamide (PAAm) (30 min for BAEC detachment) [20]. The latter design has the benefit of modulating the surface properties of specific cells, which could be useful for harvesting various types of cells. Copolymerization of IAAm with various types of hydrophilic or hydrophobic monomers (such as methacrylate (BMA) and PEG) is another approach to modify the temperature-responsive surface to control cell attachment/detachment [31][32] and cell patterning [33]. Mitsuhiro E. et al. demonstrated that grafting Poly(IAAm-co-carboxyisopropylacrylamide (CIPAAm)) on TCPS accelerated detachment of BAECs, reducing the detachment time to 35 min, compared to a control PIPAAm dish (from which BAECs took 60 min to detach) [21]. To mimic specific tissue functions, it is important to fabricate cell sheets from multiple cell types using a co-culture system. However, the proliferation and adhesion properties varied among cell types because of the differences in expression levels and types of adhesion molecules on the cell surface [34]. Therefore, the micropatterned temperature-responsive surface was designed to harvest cell sheets containing multiple cell types. An example of IAAm copolymer for cell patterning was demonstrated by Tsuda Y. et al. In this system, poly(IPAAm-co-BMA) on TCPS dishes was grafted on TCPS dished using the electron beam irradiation method, and cell attachment/detachment could be modulated by varying the BMA content [35].
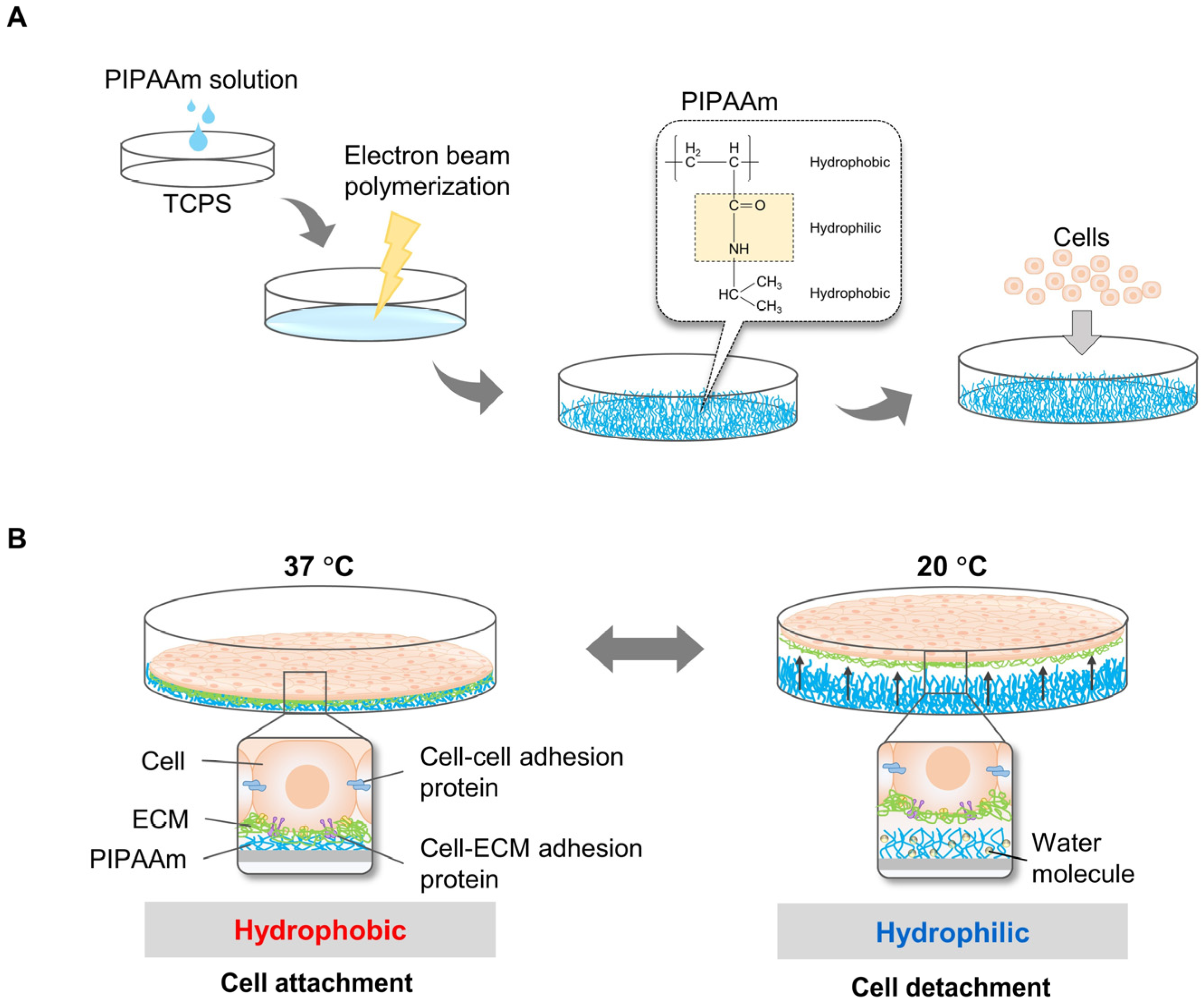
Figure 1. Principles of cell sheet harvesting using PIPAAm grafted surface. PIPAAm was grafted on the TCPS surface by electron beam polymerization before cells were allowed to grow on the surface. (A) Cells attached and proliferated on the PIPAAm cultured surface incubated at 37 °C, higher than the LCST of 32 °C. To harvest the cell sheet, cells were incubated at 32 °C below the LCST. PIPAAm was hydrophilic at this temperature (B).
PIPAAm-grafted surfaces have shown to be non-toxic and biocompatible when tested in vitro with various types of cells, including endothelial cells, epithelial cells, smooth muscle cells, and fibroblasts [36][37]. Harvesting of co-culture cell sheets has been successfully demonstrated using a PIPAAm-grafted system [38][39]. Numerous studies have successfully demonstrated the fabrication of cell sheets from various types of cells using a temperature-responsive PIPAAm-based polymer for preclinical and clinical trials. This system provides a highly reproducible and stable surface for temperature-responsive cell cultures.
However, grafting a PIPAAm-based polymer onto culture surfaces is complicated and requires special equipment, which is technically difficult and very costly. TCPS culture surfaces grafted with PIPAAm are already commercially available, making the preparation of cell sheets with a few steps, but these culture surfaces are much more expensive than general cell-culture vessels.
2.2. Temperature-Responsive System Using Methylcellulose (MC)
MC is a water-soluble polysaccharide derived from cellulose by partially substituting hydrophilic hydroxyl groups with methoxy groups. The phase transition between the MC–water solution and the gels is characterized by the LCST, which varies depending on the concentration of MC in the aqueous solution [40] and the addition of salts [41]. This property is associated with a change in hydrophilicity at temperatures below the LCST, hydrogen bonding between water and MC hydroxyl groups, hydrophobicity at temperatures above the LCST, dehydration via the exposure of methoxy groups, and stronger interactions among them. Additionally, MC is easy to use, inexpensive, and biocompatible. These features make MC a promising material for cell sheet fabrication and tissue engineering.
In addition, MC-based temperature-responsive surfaces have been designed to overcome the challenges of the complicated and time-consuming process of electron beam irradiation for PIPAAm grafting. In 2006, Chen et al. demonstrated a simple and inexpensive method to fabricate a cell sheet using an 8% w/v MC solution mixed with distinct salts (e.g., Na2SO4 and phosphate-buffered saline) on TCPS dishes at room temperature (~20 °C), which subsequently gelled at 37 °C (MC hydrogel) (Figure 2A). The gel at 37 °C was coated with neutral aqueous collagen at 4 °C for cell attachment. Cells were allowed to grow confluently on the MC/PBS/collagen surface to form a monolayer. The cell sheets were harvested at 20 °C without proteolytic enzyme treatment (Figure 2B). Cell sheets (containing human foreskin fibroblasts) were completely removed after 20 and 10 min by shaking [22]. However, the procedure described was complex, did not produce uniform hydrogels, and was consistently unstable because the MC formulation used was too viscous to be easily manipulated. To address this challenge, the optimal composition of the MC/PBS/collagen surface was systematically investigated. The production of stable hydrogels was dependent on the molecular weight (MW) and concentration of MC, as well as the type and concentration of the added salt. The optimal combination of MC–water–salt was found to be 12–16% of MC (MW = 15,000 g/mol) in 0.15 M PBS (~150 mOsm). Following this procedure, an MC gel was formed at ~32 °C. Detachment of the entire cell sheet was completed at room temperature (30 °C) for 2–3 min. Furthermore, monolayer and thick multilayer cell sheets were successfully constructed [23].
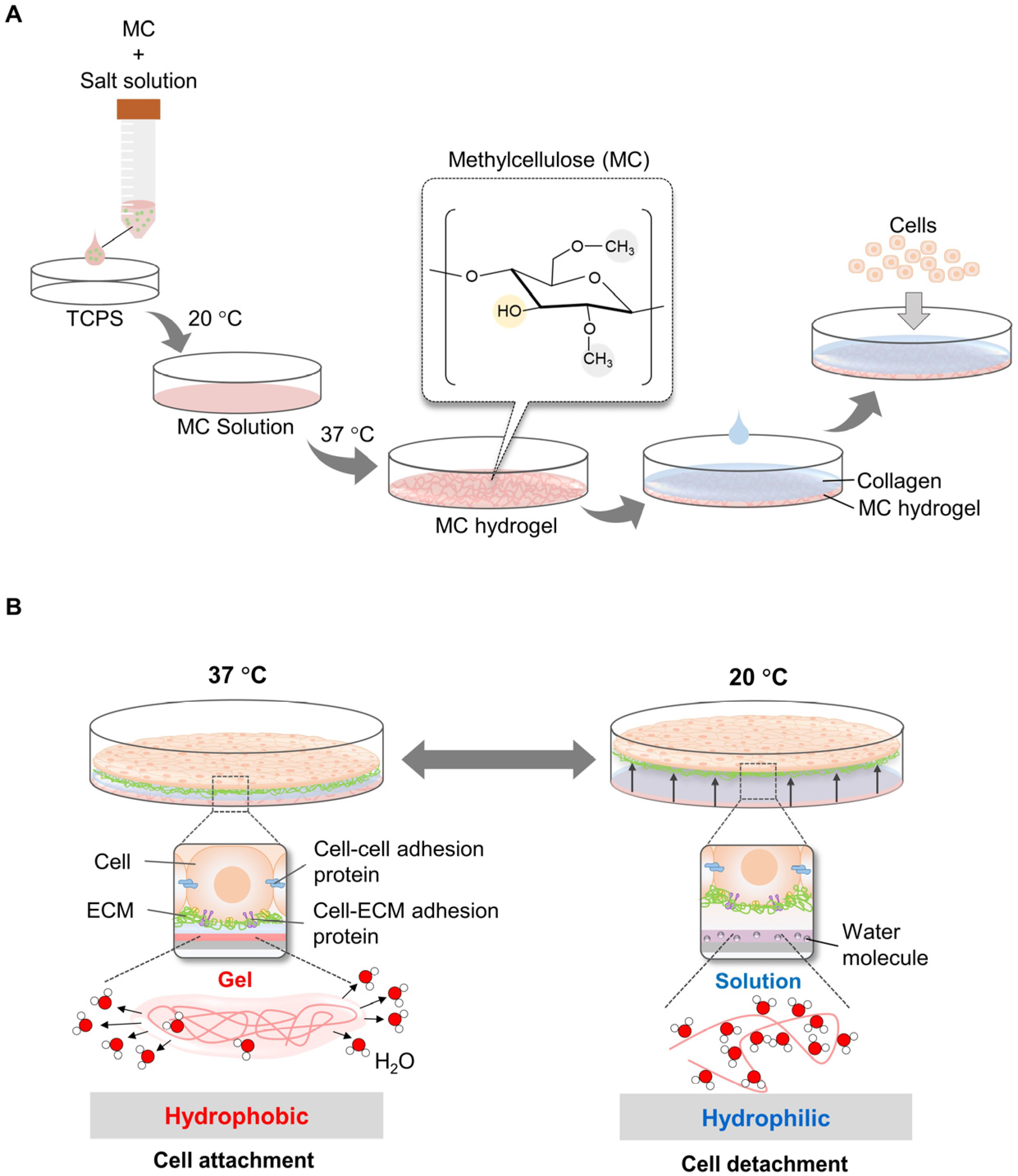
Figure 2. Principles of cell sheet harvesting using MC/collagen-coated surface. MC mixed with salt solution (Na2SO4 or PBS) was poured on the TCPS surface and gelled at 37 °C. Subsequently, neutral collagen at 4 °C was coated on the MC surface to increase cell attachment efficiency (A). Cells on MC/collagen-coated surface were grown at 37 °C, which MC was hydrophobic. By decreasing the temperature to 20 °C, MC was transformed and bonded with water molecules. As a result, cells were detached from the MC surface (B).
Similar to PIPAAm, the MC surface also provides a non-toxicity platform for various cell types, including stem cells [42]. However, a comparative study demonstrated that MC may decrease cell proliferation in cell sheets after culture for more than 2 weeks, while cells on the PIPAAm surface can continue to proliferate [42]. The advantages and disadvantages of responsive systems are listed in Table 2.
Table 2. The advantages and disadvantages of the different types of platforms for cell sheet engineering.
| Responsive Systems | Advantages | Disadvantages | Refs |
|---|---|---|---|
| PIPAAm-grafted surface |
|
|
[10][28][36][38][39][42][43][44] |
| MC-coating surface |
|
|
[22][42] |
| Ion-induced cell detachment |
|
|
[24] |
| Electro-responsive surface |
|
|
[25][45] |
| Photo-responsive surface |
|
|
[46] |
| pH-responsive system |
|
|
[47][48] |
2.3. Non-Temperature Responsive Systems Using Ion-Induced Cell Detachment
The ion-induced cell detachment method was designed as a simple isothermal system to detach cells at the desired time. This system does not require an electron beam or vapor-phase polymerization equipment or facilities to graft the cell-culture surface [49]. Since a temperature-responsive surface can damage highly sensitive cells, alter cellular metabolism, and change the cell cycle, gene expression, and function [50], an ion-induced cell detachment surface is safer for cultured cells. The principle of this system is based on the fact that cell-substrate adhesion can be regulated by modulating the surface energy of the cell-culture substrate: high surface energy promotes cell adhesion, and vice versa [51][52][53]. Therefore, this approach is composed of two platforms to generate a non-responsive cell sheet, modulate cell–substrate adhesion, and trigger the detachment of cells at the designed time without cell damage. To control cell–substrate interactions, a copolymer film comprising nonpolar hydrophobic divinylbenzene (DVB) and polar hydrophilic 4-vinylpyridine (4VP) was generated on a tissue culture surface using the initiated chemical vapor deposition (iCVD) method [54]. The surface of the copolymer film can be adjusted by controlling the input flow ratio from DVB to 4VP. These surface properties can be used to modulate the adsorption of ECM proteins, such as fibronectin, on surface-modified culture plates (Figure 3A).
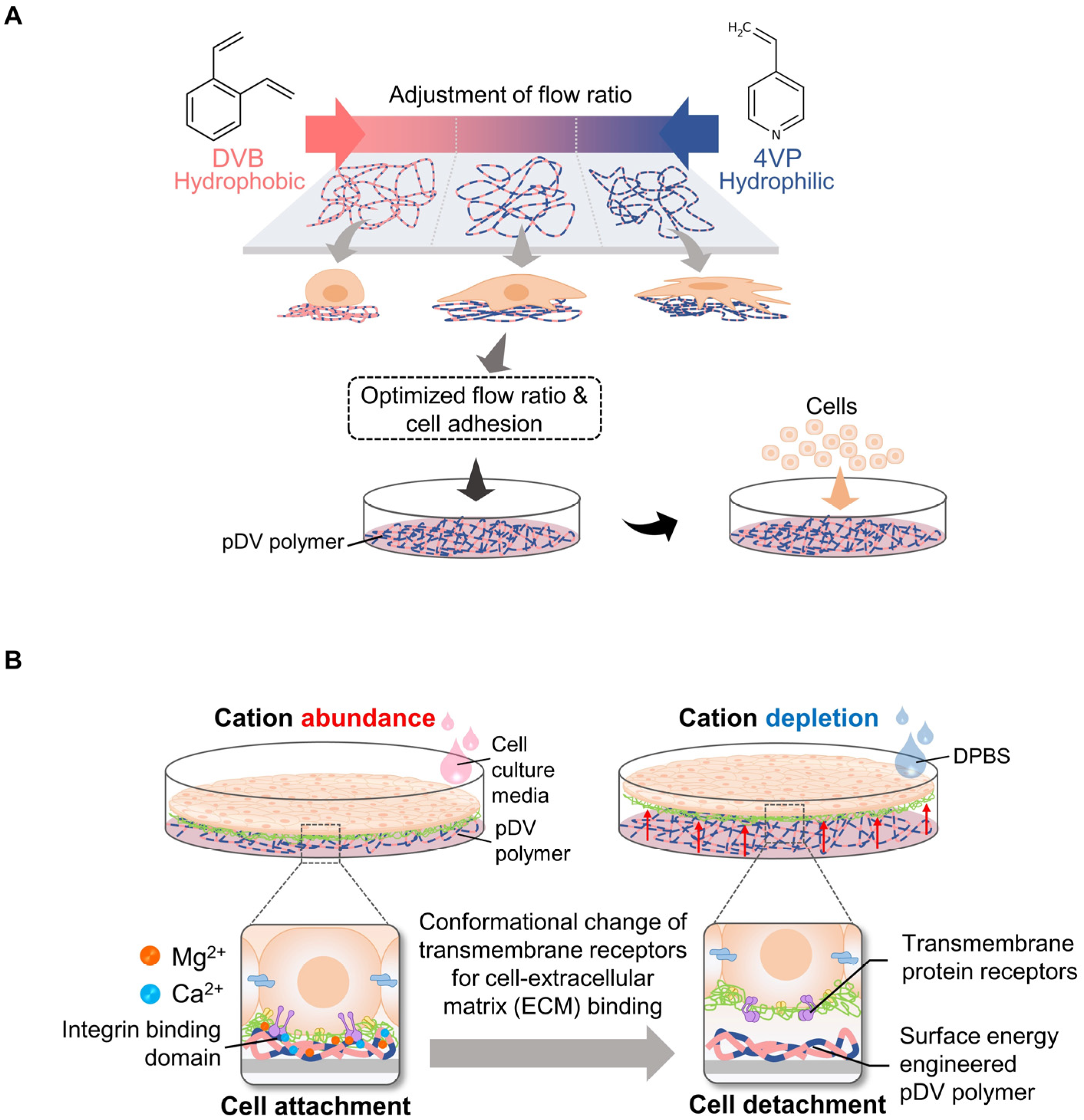
Figure 3. Principles of cell sheet harvesting using engineered pDV film coupled with divalent cation depletion. The flow ratio of DVB and 4VP was optimized for cell attachment efficiency and copolymerized on a cultured surface (A). To trigger cell detachment, the cell environment was depleted in bivalent cations (Mg2+ and Ca2+) by pouring DPBS into cell culture dishes or plates. Consequently, transmembrane proteins responsible for cell ECM occurring were conformationally changed and unbound from the binding domain. As a result, cells were detached from the culture surface (B).
The trigger method to release cell sheets from the culture surface involves the conformational change of integrin, which is a family of cell surface proteins that modulates cell–substrate or cell–ECM interactions, upon the depletion of divalent cations such as Ca2+ and Mg2+ [55]. Therefore, cell sheets can be harvested under isothermal conditions at the desired time by adding Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ and Mg2+ (Figure 3B). The detachment of the cell sheet could be accelerated to within 100 s. In this study, there were five types of cells, including thermosensitive cell types (myoblast cell line (C2C12) and fibroblast cell line (NIH-3T3 cells)) and non-thermosensitive cell types (mesenchymal stem cells (MSCs)), normal human dermal fibroblasts (NHDFs) and endothelial cell line (C166)), used to evaluate the efficiency of the developed method. These cells were completely detached as intact cell sheets. In addition, this non-responsive system has been used to fabricate monolayer and thick multilayer cell sheets in both in-vitro and in-vivo studies. Moreover, the previous study confirmed that the pDV surface did not significantly affect cell–cell adhesion and cell viability [24].
2.4. Non-Temperature-Responsive Systems Using Electro-Responsive Surfaces
Another common approach for recovering cell sheets is to utilize an electro-responsive surface. In principle, electrical stimulation is the signal that triggers cell detachment in this system. The major component of this system is a self-assembled monolayer (SAM) of alkanethiolates on gold connected to immobilizing peptide ligands containing Arg-Gly-Asp (RGD), a binding site for cell adhesion. The types of immobilizing ligands can be designed to adapt to various cells for adhesion. When a negative electrical potential is applied to the gold film, the monomer is oxidized, resulting in the rapid release of the immobilized ligands [45]. The detachment of the cell sheets from these surfaces was completed by applying −1.0 V, and cells became completely detached within 10 min, faster than on temperature-responsive surfaces. Electro-responsive systems are adaptable and are comprised of different cell-culture surfaces, allowing the creation of varying cell sheet sizes with different forms and thicknesses [56].
Enomoto et al. demonstrated the use of an electro-responsive surface to prepare fibroblast sheets with a thickness of ~50 μm. The oligopeptide CCRRGDWLC, which contains RGD, was designed for ligand immobilization. Fibroblasts grew rapidly on the membrane for 14 days, and the thickness of the cell sheet became ~60 μm, which is almost three times thicker than that of cells cultured in conventional cell-culture plates. Subsequently, the stacking of these cell sheets to up to five layers established multiple thick cell sheets with a total thickness of more than 200 μm [57]. However, a necrotic core began to develop in these cell sheets. This problem can be overcome by generating a continuous flow of the culture medium around the stacked sheets to provide better oxygenation and nutrient provision [57].
Another experiment to create thick tissues was conducted by Kobayashi et al. They combined an electric-responsive platform with microstereolithography to create a thick three-dimensional tissue with a complicated shape. The idea behind these types of cell sheets is to apply them to repair the intestinal wall, as they can be made to fit their anatomical features in a precise manner. The advantage of these 3D cell sheets is their use for the repair of more complex structures and the regeneration of more complex organs [25].
2.5. Other Systems
A photo-responsive system requires a fabrication method which uses light as a trigger to control wettability. Light can illuminate and reversibly change the conformation of photo-responsive materials due to changes in various properties, including magnetism, fluorescence, and wettability. Among these properties, light has attracted attention as a cell-adhesion controller. Metal oxides, primarily zinc oxide (ZnO) and titanium dioxide (TiO2), are the most widely used photo-responsive materials. Hong et al. demonstrated the use of a photo-responsive cell sheet system. They designed a cell sheet system by coating a TiO2 nanodot-coated quartz substrate onto a cell-culture plate, and pre-osteoblastic cells were seeded until confluency. After UV-light (365 nm) illumination, the cell sheets were completely detached within 20 min [46]. This evidence demonstrates that a photo-responsive system is a promising method for harvesting cell sheets.
Furthermore, pH-responsive systems have been widely used in drug delivery systems because of the pH variability in the human body, which can control the release of drugs to the target area. Classic examples of this system include cancer-drug delivery systems. pH-responsive systems allow anti-cancer drugs to be released at the tumor site, where the pH is approximately 6.5–7.2, while the pH of the physical condition is 7.4. However, few studies have used pH-responsive systems for cell sheet fabrication due to the limited range of pH values (6.8–7.4) allowed for normal cell function. Chen et al. developed a pH-responsive chitosan surface to control cell detachment within a small pH range. HeLa cells attached to the surface of chitosan at pH 6.99 and 7.20. After the pH was increased to 7.65, almost all cells detached from the surface within 1 h and survived [48].
Various responsive systems have been developed to enable the detachment of confluent cell sheets; however, the limitations of these systems require further study. These include the evaluation of the potential of these systems with numerous cell types and their extension for various applications. Some cells that attach firmly to a surface may have a longer detachment time. In addition, commercially responsive surfaces are already available for cell sheet detachment; however, they are costly, especially when thick 3D tissue constructs need to be fabricated using a large number of cell sheets. Another challenge is that most current fabrication technologies remain inaccessible and complicated. Therefore, the development of simple and economical fabrication methods for responsive surfaces is necessary and will greatly encourage researchers to exploit cell sheet engineering.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10020211
References
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing Into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202.
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65.
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13.
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015, 79, 991–999.
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020, 11, 19.
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196.
- Sato, M.; Yamato, M.; Hamahashi, K.; Okano, T.; Mochida, J. Articular Cartilage Regeneration Using Cell Sheet Technology. Anat. Rec. 2014, 297, 36–43.
- Takagi, R.; Yamato, M.; Kanai, N.; Murakami, D.; Kondo, M.; Ishii, T.; Ohki, T.; Namiki, H.; Yamamoto, M.; Okano, T. Cell sheet technology for regeneration of esophageal mucosa. World J. Gastroenterol. 2012, 18, 5145–5150.
- Ito, M.; Shichinohe, H.; Houkin, K.; Kuroda, S. Application of cell sheet technology to bone marrow stromal cell transplantation for rat brain infarct. J. Tissue Eng. Regen. Med. 2017, 11, 375–381.
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chemie Rapid Commun. 1990, 11, 571–576.
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362.
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Scaffold-free tissue engineering using cell sheet technology. RSC Adv. 2012, 2, 2184–2190.
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858.
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac Cell Sheet Transplantation Improves Damaged Heart Function via Superior Cell Survival in Comparison with Dissociated Cell Injection. Tissue Eng. Part A 2011, 17, 2973–2980.
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Okano, T. Rapid cell sheet detachment from Poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J. Biomed. Mater. Res. 2000, 50, 82–89.
- Kikuchi, A.; Okuhara, M.; Karikusa, F.; Sakurai, Y.; Okano, T. Two-dimensional manipulation of confluently cultured vascular endothelial cells using temperature-responsive poly(N-isopropylacrylamide)-grafted surfaces. J. Biomater. Sci. Polym. Ed. 1998, 9, 1331–1348.
- Hyeong Kwon, O.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials 2003, 24, 1223–1232.
- Tang, Z.; Akiyama, Y.; Yamato, M.; Okano, T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials 2010, 31, 7435–7443.
- Kim, S.J.; Kim, W.I.; Yamato, M.; Okano, T.; Kikuchi, A.; Kwon, O.H. Successive grafting of PHEMA and PIPAAm onto cell culture surface enables rapid cell sheet recovery. Tissue Eng. Regen. Med. 2013, 10, 139–145.
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell-sheet recovery from a surface successively grafted with polyacrylamide and poly(N-isopropylacrylamide). Acta Biomater. 2014, 10, 3398–3408.
- Ebara, M.; Yamato, M.; Hirose, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Copolymerization of 2-Carboxyisopropylacrylamide with N-Isopropylacrylamide Accelerates Cell Detachment from Grafted Surfaces by Reducing Temperature. Biomacromolecules 2003, 4, 344–349.
- Chen, C.-H.; Tsai, C.-C.; Chen, W.; Mi, F.-L.; Liang, H.-F.; Chen, S.-C.; Sung, H.-W. Novel Living Cell Sheet Harvest System Composed of Thermoreversible Methylcellulose Hydrogels. Biomacromolecules 2006, 7, 736–743.
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Methylcellulose Based Thermally Reversible Hydrogel System for Tissue Engineering Applications. Cells 2013, 2, 460–475.
- Baek, J.; Cho, Y.; Park, H.-J.; Choi, G.; Lee, J.S.; Lee, M.; Yu, S.J.; Cho, S.; Lee, E.; Im, S.G. A Surface-Tailoring Method for Rapid Non-Thermosensitive Cell-Sheet Engineering via Functional Polymer Coatings. Adv. Mater. 2020, 32, e1907225.
- Kobayashi, Y.; Cordonier, C.E.J.; Noda, Y.; Nagase, F.; Enomoto, J.; Kageyama, T.; Honma, H.; Maruo, S.; Fukuda, J. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci. Rep. 2019, 9, 10415.
- Kenji, K.; Fujishige, S.; Ando, I. Solution properties of poly(N-isopropylacrylamide) in water. Polym. J. 1990, 22, 15–20.
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031.
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin Poly(N-isopropylacrylamide) Grafted Layer on Polystyrene Surfaces for Cell Adhesion/Detachment Control. Langmuir 2004, 20, 5506–5511.
- Yang, J.; Yamato, M.; Shimizu, T.; Sekine, H.; Ohashi, K.; Kanzaki, M.; Ohki, T.; Nishida, K.; Okano, T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials 2007, 28, 5033–5043.
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303.
- Lutz, J.-F. Thermo-Switchable Materials Prepared Using the OEGMA-Platform. Adv. Mater. 2011, 23, 2237–2243.
- Akiyama, Y. Design of Temperature-Responsive Cell Culture Surfaces for Cell Sheet Engineering. Cyborg Bionic Syst. 2021, 2021, 5738457.
- Yamato, M.; Konno, C.; Utsumi, M.; Kikuchi, A.; Okano, T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials 2002, 23, 561–567.
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184.
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Umezu, M.; Okano, T. Control of cell adhesion and detachment using temperature and thermoresponsive copolymer grafted culture surfaces. J. Biomed. Mater. Res. Part A 2004, 69A, 70–78.
- Takezawa, T.; Mori, Y.; Yoshizato, K. Cell Culture on a Thermo-Responsive Polymer Surface. Biotechnology 1990, 8, 854–856.
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19.
- Thummarati, P.; Kino-Oka, M. Effect of Co-culturing Fibroblasts in Human Skeletal Muscle Cell Sheet on Angiogenic Cytokine Balance and Angiogenesis. Front. Bioeng. Biotechnol. 2020, 8, 578140.
- Harimoto, M.; Yamato, M.; Hirose, M.; Takahashi, C.; Isoi, Y.; Kikuchi, A.; Okano, T. Novel approach for achieving double-layered cell sheets co-culture: Overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J. Biomed. Mater. Res. 2002, 62, 464–470.
- Hussain, S.; Keary, C.; Craig, D.Q.M. A thermorheological investigation into the gelation and phase separation of hydroxypropyl methylcellulose aqueous systems. Polymer 2002, 43, 5623–5628.
- Zheng, P.; Li, L.; Hu, X.; Zhao, X. Sol-gel transition of methylcellulose in phosphate buffer saline solutions. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1849–1860.
- Forghani, A.; Kriegh, L.; Hogan, K.; Chen, C.; Brewer, G.; Tighe, T.B.; Devireddy, R.; Hayes, D. Fabrication and characterization of cell sheets using methylcellulose and PNIPAAm thermoresponsive polymers: A comparison Study. J. Biomed. Mater. Res. Part A 2017, 105, 1346–1354.
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma Polymerized N-Isopropylacrylamide: Synthesis and Characterization of a Smart Thermally Responsive Coating. Biomacromolecules 2001, 2, 32–36.
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245.
- Yeo, W.-S.; Hodneland, C.D.; Mrksich, M. Electroactive Monolayer Substrates that Selectively Release Adherent Cells. Chembiochem 2001, 2, 590–593.
- Hong, Y.; Yu, M.; Weng, W.; Cheng, K.; Wang, H.; Lin, J. Light-induced cell detachment for cell sheet technology. Biomaterials 2013, 34, 11–18.
- Guillaume-Gentil, O.; Semenov, O.V.; Zisch, A.H.; Zimmermann, R.; Voros, J.; Ehrbar, M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials 2011, 32, 4376–4384.
- Chen, Y.-H.; Chung, Y.-C.; Wang, I.-J.; Young, T.-H. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials 2012, 33, 1336–1342.
- Nash, M.E.; Healy, D.; Carroll, W.M.; Elvira, C.; Rochev, Y.A. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. J. Mater. Chem. 2012, 22, 19376–19389.
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited Review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742.
- Lim, J.Y.; Shaughnessy, M.C.; Zhou, Z.; Noh, H.; Vogler, E.A.; Donahue, H.J. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 2008, 29, 1776–1784.
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941.
- Decuzzi, P.; Ferrari, M. Modulating cellular adhesion through nanotopography. Biomaterials 2010, 31, 173–179.
- Joo, M.; Shin, J.; Kim, J.; You, J.B.; Yoo, Y.; Kwak, M.J.; Oh, M.S.; Im, S.G. One-Step Synthesis of Cross-Linked Ionic Polymer Thin Films in Vapor Phase and Its Application to an Oil/Water Separation Membrane. J. Am. Chem. Soc. 2017, 139, 2329–2337.
- Lange, T.S.; Bielinsky, A.K.; Kirchberg, K.; Bank, I.; Herrmann, K.; Krieg, T.; Scharffetter-Kochanek, K. Mg2+ and Ca2+ Differentially Regulate β1 Integrin-Mediated Adhesion of Dermal Fibroblasts and Keratinocytes to Various Extracellular Matrix Proteins. Exp. Cell Res. 1994, 214, 381–388.
- Inaba, R.; Khademhosseini, A.; Suzuki, H.; Fukuda, J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 2009, 30, 3573–3579.
- Enomoto, J.; Mochizuki, N.; Ebisawa, K.; Osaki, T.; Kageyama, T.; Myasnikova, D.; Nittami, T.; Fukuda, J. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regen. Ther. 2016, 3, 24–31.
This entry is offline, you can click here to edit this entry!
