Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medical Laboratory Technology
Several bioactive compounds of marine origin are being studied, and among these, marine collagen represents one of the most attractive bio-resources, given its use in various disciplines, such as clinical applications, cosmetics, the food sector, and many other industrial applications. This review aims to provide a current overview of marine collagen applications in the pharmacological and biomedical fields, regenerative medicine, and cell therapy.
- marine collagen
- jellyfish
- tissue
1. Use of Marine Collagen in Blends
A growing number of biomimetic composites based on collagen from marine origin are being developed and display very promising properties [81]. Marine biomaterials based on polysaccharides (chitin, fucoidan, alginate, etc.) and collagen are currently being thoroughly investigated for the biomedical industry, thanks to their biocompatibility in many applications and their specific properties. Collagen is generally widely used in various sectors; it is possible to improve its characteristics and mechanical properties, to make it more suitable for use in biomedical fields. Blends can be prepared based on synthetic and natural polymers, leading to the development of a new class of materials (biocomposites), characterized by improved mechanical properties and biocompatibility, when compared with materials of a single polymer [82]. In this regard, much research has been undertaken on chitosan, marine collagen- and alginate-based composites. Chitosan can be combined with collagen [83], or with collagen and hydroxyapatite [84,85]; chitosan/collagen cross-linked hybrid scaffolds possess better mechanical and degradation properties than their unitary systems separately, especially at the optimal ratio of 50/50. Thanks to their good cytocompatibility, those scaffolds can be used for the nerve tissue regeneration, as they promote the attachment, migration and proliferation of Schwann cells [86]. On the other hand, the combination of jellyfish collagen with functionalized denatured fish collagen (gelatin) permits the generation of collagen-based marine hydrogel (MCh), which has higher stability and is useful as an injectable cell delivery system for the cellular repair of cartilage in clinical applications.
Shark-skin collagen is known to promote osteoblast growth and collagen synthesis in bone cells [87]. When this collagen is further mixed with the calcium phosphate of shark teeth to form a 3D composite scaffold, it better supports the attachment and proliferation of osteoblast-like cells [88].
These and other examples demonstrate how the use of blends can support the poor mechanical properties of marine collagen, improving its important biological functions (Figure 1).
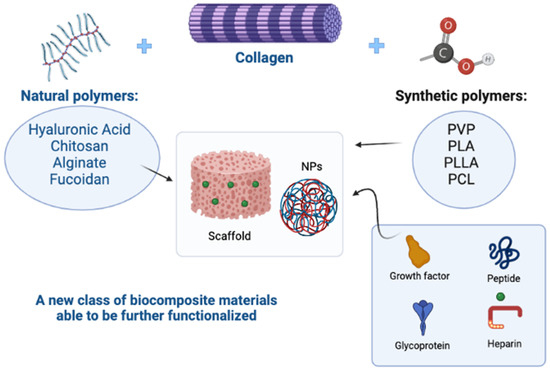
Figure 1. The use of synthetic and natural polymers in blends has led to the development of a new class of materials that support the poor mechanical properties of natural polymers, improving their important biological functions. In addition, scaffolds and nanoparticles (NPs) can be further functionalized with glycosaminoglycans such as heparin, glycoproteins, peptides, or growth factors, to improve the biocompatibility of the devices (Polyvinylpyrrolidone-PVP; Polylactic acid-PLA/PLLA; Polycaprolactone-PCL). Created with BioRender.com.
2. Marine Collagen and Pharmacological Applications
In addition to the numerous biological properties of marine collagen, such as the ability to stimulate the regeneration of tissues such as bones and skin, and to give elasticity to tendons, etc., collagen hydrolysate and its low molecular weight peptides also show remarkable therapeutic properties and are of great interest for pharmacological and biomedical applications, as an excellent alternative to mammalian collagen. Many researchers have found that low-molecular-weight collagen peptides (di- and tripeptides), particularly those with Pro or Hyp C-terminal residues, have numerous bioactivities, including antibacterial [89], antioxidant [90], anti-inflammatory, immune-modulatory [91] and ACE-inhibitory properties [92,93]. Furthermore, another important property of collagen peptides is the ability to regulate lipid metabolism and cholesterol levels, with lipid-lowering and antiobesity effects [94], and to improve wound healing, applying tilapia collagen to a wound stimulated healing by increasing keratinocyte proliferation, fibroblast and myofibroblast differentiation, and ECM production [95]. Morishige and coworkers have demonstrated that collagen extracts obtained from the giant edible jellyfish Nemopilema nomurai, stimulate the production of immunoglobulins (Igs) and cytokines by human hybridoma cells and peripheral blood lymphocytes. Their data showed an in vivo immunostimulatory effect of jellyfish collagen, without any allergic complications [96]. Preliminary data from an in vitro study, demonstrated that the purified peptide fraction of collagen, obtained from fish skin hydrolysate, had an interesting anti-inflammatory property in the cellular microenvironment and could be used as a nutraceutical supplement [97,98]. Tomosugi and coworkers have shown that, in a rabbit model, the oral administration of the tripeptide component of collagen (CTP) results in its selective absorption into connective tissues; according to the results of these reports, CTP should have utility as a functional food for the prevention and treatment of atherosclerosis [99].
Decreases in atherosclerotic plaque area, serum total cholesterol levels, and the numbers of macrophages and smooth muscle cells in atherosclerotic plaques, have also been observed [99,100]. Furthermore, Gly-Pro-Hyp, the main component of CTP, inhibits dipeptidyl peptidase-IV activity, indicating that CTP may have potential utility in the prevention of diabetes [101,102].
There are several supplements in capsules used for the oral administration of fish collagen peptides, mainly used to counteract aging processes, in the cosmetic field. Low-molecular-weight collagen peptides can cross the intestinal barrier and enter the bloodstream in significant quantities. They can be absorbed intact from the intestine and resist degradation by plasma peptidases. This ability gives collagen peptides an effective biological function since they reach the target sites in an active form [103]. Following oral intake, collagen peptides are known to increase fibroblast production, activate multiple biochemical pathways, including the increase of hyaluronic acid production in dermal fibroblasts, and improve the water content of the skin. Commercially available marine collagen peptides have recently been clinically proven to be safe and effective for skin beauty, particularly when combined with skin-targeted plant-derived antioxidants, such as coenzyme Q10 [59]. However, oral administration of these peptides also shows other beneficial effects on the body. Marine collagen has been reported to reduce the production of proinflammatory cytokines such as COX-2, NO, MMP-13, and CTX-II [104], and is able to protect thymic epithelial cells (TECs) from cytotoxic and oxidative damage induced by cisplatin administration, which is used in the treatments of many cancers, due to its inhibition of the MAPK signal transduction pathway [105]. In addition to their antioxidant, antithrombotic, anticoagulant, anti-inflammatory, antiproliferative, antihypertensive, antidiabetic, and cardioprotective properties, marine-sourced biocompounds have been investigated for their neuroprotective potential [106]. Neurodegeneration is a complex, progressive multifactorial process that leads to the loss and death of neuronal structures in the nervous system; it is associated with the accumulation of insoluble deposits of protein and peptide aggregates, generally containing misfolded proteins, in different areas of the brain and spinal cord [107]. As oxidative stress has been considered to play an essential role in the onset and progression of neurodegeneration, there has been a significant scientific focus on the development of antioxidant compounds for neuroprotection [108]. Marine polysaccharides, in particular, chitosan derived from the hydrolysis of chitin, alginate extracted from algae [109,110], marine glycosaminoglycans such as hyaluronic acid (HA), chondroitin sulfate (CS), heparin and heparan sulfate (HS) obtained from mussels, codfish bones, tuna eyeballs, and shark fins [111,112], and marine glycoproteins [113] and glycolipids [114,115], are the most physiologically important biocompounds involved in neuroprotective activities that can be extracted from marine sources.
It is therefore evident that marine collagen peptides exhibit interesting biological activities and can be efficiently used for the treatment of various pathologies: skin ageing, bone defects, sarcopenia, wound healing, periodontal disease, gastroesophageal reflux, osteoarthritis and rheumatoid arthritis [116].
The abundance of collagen in marine organisms (fish, starfish, sponges, jellyfish, etc.), the resistance of some collagen-derived peptides to gastrointestinal digestion, as well as their ability to reach the bloodstream intact [59], suggest that marine collagen could be an interesting source of bioactive peptides, with promising pharmacological applications as functional foods and in drug delivery.
3. Marine Collagen and Biomedical Applications
The polymeric nature of collagen, as well as its low immunogenicity, makes it an excellent material for use as a scaffold, and for the production of smart nanocarriers for gene and/or cell delivery for tissue engineering applications. The biocompatibility between human and jelly-derived collagen suggests applications in the stimulation of tissue regeneration. The polymers used for scaffold preparation are mainly of biological origins, such as chitosan, silk fibroin, hyaluronic acid, collagen, and many others [117]; however, as mentioned above, the combination of organic and synthetic polymers gives rise to scaffolding characteristics combining the mechanical strength of synthetic materials and the biocompatibility of the natural materials. The most common synthetic biodegradable polymers in medical applications are poly (α-hydroxy acids), including poly-glycolic acid (PGA), poly-lactic acid (PLA), and polydioxanone (PDS). PLA, PGA, and their copolymers have been investigated for a higher number of applications compared with other degradable polymers [118,119,120]. The high interest in these materials is based on their good processability and mechanical properties, but above all on their ability to safely degrade without releasing toxic compounds, and on their high degree of biocompatibility [121]. Many synthetic polymers are used to produce three-dimensional and highly porous scaffolds, used specifically for bone and cartilage regeneration [122,123,124,125]. Bone tissue engineering is a promising strategy for the treatment of bone-related disorders, including osteoporosis and bone defects, promoting bone regeneration through the coordinated integration of stem cells, biomaterials, and bone-inducing factors. Although biomaterials represent the basic component of scaffolds in bone tissue engineering, they are often structurally modified or surface-functionalized to increase their potential. The functionalization of polymeric scaffolds with organic polymers, such as collagen or proteoglycans, is a promising approach to improve cytocompatibility. Organic polymers, isolated directly from the extracellular matrix, contain a multitude of surface ligands (fibronectin, laminin, vitronectin, etc.) and arginine–glycine–aspartic acid-containing peptides that promote cell adhesion. In tissue engineering, the combination of organic bioactive molecules and synthetic polymers gives rise to scaffolds characterized simultaneously by the mechanical strength of synthetic materials and the biocompatibility of natural materials [126].
Marine collagen also has applications in vascular tissue engineering. There is extensive evidence regarding the role played by the extracellular matrix (ECM), of which collagen Type I is among the main components, in driving capillary morphogenesis through sustained signaling, resulting in persistent endothelial cells (ECs) cytoskeletal reorganization and changes in cell form. A direct interaction between ECs and the ECM is necessary during angiogenesis, especially during the sprouting of new blood vessels from the existing vasculature [127,128]. Marine collagen, as well as mammalian ones, can contribute to the architecture and strength of tissues, interacting with cells through numerous receptors, and promoting cell growth, differentiation, and migration [123]. In a tumor context, collagen remodeling (degradation and redeposition) strongly influences tumor infiltration, angiogenesis, and migration; its reorganization at the tumor-stromal interface facilitates local invasion [129,130,131]. Paradiso and coworkers demonstrated that R. pulmo collagen is effective in manufacturing 3D devices, such as sponges, where it mimics the complexity of tissue architecture. OvCa cells migrated and differentiated within R. Pulmo collagen 3D scaffolds, confirming its suitability for advanced cell culture applications, and providing an excellent alternative to mammalian collagen sources for human cell culture [132].
Another biomedical application, in which the influence of collagen is widely documented, is the regeneration of cartilage tissues [133,134,135,136]. Cartilage is a connective tissue composed of sparsely distributed chondrocytes, embedded within a dense extracellular matrix primarily composed of type II collagen and proteoglycans. Unlike other tissues, articular cartilage is avascular and exhibits poor capability for self-repair; consequently, cartilage injuries are difficult to treat [137]. The transplantation of mesenchymal stem cells is a favorable approach due to their high proliferative activity and their capacity to differentiate into chondrocytes, which are responsible for cartilage synthesis and maintenance [138]. However, the most well-known cell-based repair strategy for large cartilage injuries is autologous chondrocyte implantation, which uses in vitro enriched chondrocytes from cartilage biopsy. Unfortunately, in monolayer culture, isolated chondrocytes lose their differentiated phenotype and shift towards a fibroblast-like phenotype [139]. Preserving the differentiated state, which ensures the ability to regenerate damaged cartilage, represents the main challenge during in vitro culturing. Three-dimensional (3D) scaffolds have the potential to preserve the phenotype of chondrocytes; particularly, hydrogels containing highly hydrated 3D networks are highly recommended because of their similarity to native cartilage [140]. Among these, the use of a collagen-based formulation of a marine hydrogel (MCh) has been proposed; it can cross-link with the cells trapped inside it, by in situ injection in the presence of H2O2 and horseradish peroxidase (HPR), without any cytotoxic effects. MCh can maintain the chondrocyte phenotype in vitro, compared to other 3D collagen hydrogels [66]. Although the data are still preliminary, MCh appears to have enormous potential as an injectable chondrocyte delivery system for cartilage repair, in both preclinical and clinical trials [66]. Many data in the literature indicate the use of marine collagen for a variety of applications, such as dental tissue engineering [141,142], oral mucosa regeneration [143], spinal cord injury repair and nerve regeneration [144,145], skin tissue engineering and wound healing [146,147,148], corneal tissue engineering [149], and many others. All these studies show that marine-derived collagen is a promising tool for wide-ranging clinical applications, including as a drug delivery system for cancer and other diseases.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28031152
This entry is offline, you can click here to edit this entry!
