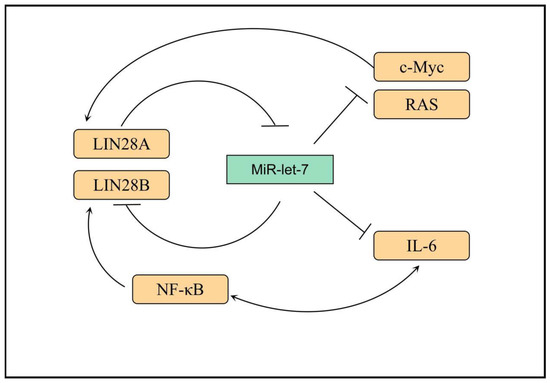
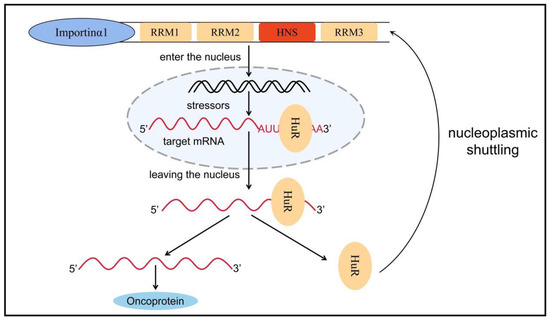
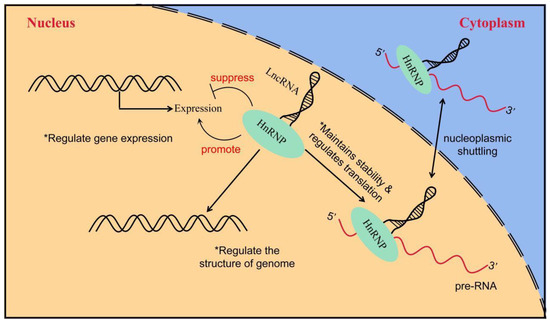
6. Others
Although four types of RBPs have been listed for their roles and functions in bladder cancer, more RBPs have been identified and reported in bladder cancer, including insulin-like growth factor messenger RNA binding protein 3 (IGF2BP3), nucleolin (NCL), and quaking (QKI). Moreover, it is reported that Fragile X-related gene 1 (FXR1) was identified as a novel cancer driver gene in urothelial carcinoma of the bladder (UCB) [
132]; circ-SLC38A1 promotes BC cells invasion in vitro and lung metastasis in vivo in mice [
133].
6.1. IGF2BP3
IGF2BP3 is a member of the IGF2BP family, and its members all contain 2 RRM and 4 KH domains in their structure; these 4 KH domains are all binding GGC sequences [
134]. Under normal or stress conditions, members of the IGF2BP family can act on target mRNAs in an N6-methyladenosine-dependent manner, promote their stability, and increase their intracellular content, thereby affecting gene expression [
135,
136]. However, currently, only IGF2BP3 has been reported in bladder cancer. The chromosomal localization of IGF2BP3 is 7p15.3.IGF2BP3, which is a typical multi-domain RNA-binding protein with specificity and diversity in recognizing targets and provides a good paradigm for the multivalent interactions of multi-domain RNA-binding proteins [
137].
Compared with IGF2BP3, which has been widely reported in a variety of tumors such as breast, liver, and gastrointestinal tract tumors, pancreatic cancer, and lung cancer [
138], knowledge on its role in bladder cancer is limited. Regardless, IGF2BP3 is highly expressed in bladder cancer patients and independently associated with bladder cancer recurrence, cancer-specific mortality, and all-cause mortality [
139]. Knocking down the expression of IGF2BP3 in bladder cancer can increase apoptosis and induce cell cycle arrest, implying that it originally promoted cell proliferation via inhibiting apoptosis and regulating cell cycle [
140].
6.2. NCL
NCL is one of the abundant proteins in the nucleolus, and it is widely distributed in the nucleolus, nucleoplasm, cytoplasm, and cell membrane of eukaryotic cells, participating in a variety of biological processes [
141]. NCL is mainly distributed in the nucleus, is responsible for the transcription of rDNA, and has various roles in the biogenesis of ribosomes, including RNA polymerase I transcription, pre-rRNA processing, and ribosome assembly [
142]. The human NCL gene is a haploid genome located on 2q12-qter, consisting of 14 exons and 13 introns [
143]. The N-terminal domain of the NCL is majorly involved in DNA regulation and protein-to-protein reactions, and this region also contains a variety of highly phosphorylated sites that can be involved in cell cycle regulation [
143]. The central domain holds four RRMs that bind to and regulate the transcription of specific mRNAs [
144]. The C-terminal sequence and arrangement of NCL is not conserved, and its length is variable, usually interspersed with a large number of glycine, arginine, and phenylalanine residues; its main function is to help NCL interact during larger or more complex RNA localization [
145]. NCL is highly expressed in a variety of tumors [
146]; besides promoting tumor proliferation [
147,
148,
149], it is an important anti-apoptotic protein that maintains tumor cell survival [
143,
150,
151,
152,
153]. NCL has also been reported to be involved in various processes, such as angiogenesis [
154,
155,
156], infiltration, and metastasis [
157,
158,
159,
160] of tumors.
In bladder cancer, increased expression of NCL boosts its aggressiveness and promotes its pulmonary metastasis [
161], whereas blocking NCL expression in bladder cancer can inhibit bladder cancer proliferation and invasion [
162,
163,
164]. However, the target mRNAs of NCL in bladder cancer, such as Rho factor [
161,
162] and/or MMP-2 [
163], are also diverse. In addition, NCL has the potential to promote tumor proliferation by promoting the function of epidermal growth factor receptor [
164,
165]. The role played by NCL in bladder cancer is far less clearly explained than other tumors, and this area needs more research input. Regardless, the treatment targeting NCL has great potential in bladder cancer.
6.3. QKI
QKI protein is a subfamily of the signal transduction and activation of RNA (STAR) family [
166]. The STAR protein family plays an important role in embryogenesis, tissue, and organ development [
167,
168]. The human Qki gene is localized on chromosome 6 and has nine exons, which can produce at least five transcripts by different splicing methods [
169]. Three of the main transcripts are the most important, as they are named Qki-6, Qki-6, and QkiI-7 due to their sizes (5, 6, and 7 kb, respectively). The structure of QKI proteins is highly homologous to other members of the STAR family, with similar domains: an RRM (KH domain) flanked by QUA1 and QUA2 domains [
169]. The QKI-5 protein is mainly localized in the nucleus, where it can bind to target mRNAs and retain them in the nucleus. This condition is possible because QKI-5 contains a nuclear localization signal peptide at its C-terminus, whereas QKI-6 and QKI-7, which lack this signal peptide, are mainly localized in the cytoplasm and are involved in target mRNA transport and regulate its stability [
170]. The reason why QKI proteins can bind to target mRNAs is that these mRNAs have a sequence that can be specifically bound by QKI, namely 5′-A(C/A)UAA-3′; hence, this sequence is also called a quaking response element (QRE) [
171]. Bioinformatic analysis showed that QKI can interact with more than 1000 mRNAs with QRE, and most of these are important molecules in cell-directed differentiation, proliferation, metastasis, and apoptosis [
171,
172]. QKI has a low expression in a variety of malignancies, and overexpression of QKI inhibits the proliferative, invasive, and migratory capacities of these tumors and promotes their apoptosis [
173,
174,
175,
176,
177]. Thus, QKI may play an important role as an oncogenic factor in the development of malignant tumors.
In bladder cancer, the low expression of QKI is associated with advanced tumor TNM staging and poor overall survival, whereas its overexpression inhibits the ability of bladder cancer cells to grow and invade [
178]. The oncogenic mechanism of miRNA-362-5p in bladder cancer is also related to QKI, which can promote the proliferative and invasive effects of bladder cancer via targeting binding and reducing QKI [
179]. Cancer-associated fibroblasts (CAFs) are also an important component of the tumor microenvironment, secreting microfibrillar-associated protein 5 (MFAP5), a component of elastic microfibers, which is also an oncogenic factor in a variety of tumors [
180], promoting tumor cell proliferation, invasion [
181], and recruiting new blood vessels [
182]. In bladder cancer, QKI can directly target binding to MFAP5 in CAFs and downregulate its expression, thus limiting tumorigenesis and progression [
183]. The expression level of QKI in malignant tumors can be used as an important indicator to predict the occurrence and development of cancer, and it is expected to become a new target for tumor therapy, including that of bladder cancer. However, how to apply it effectively and precisely in clinical diagnosis and treatment needs further research. In particular, the functional differences between the subtypes of QKI in tumorigenesis development are rarely reported.