Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Adipose-derived stem cells (ASCs) are a subpopulation of mesenchymal stem cells. Compared to bone marrow-derived stem cells, they can be harvested with minimal invasiveness. ASCs can be easily expanded and were shown to be able to differentiate into several clinically relevant cell types. Therefore, this cell type represents a promising component in various tissue engineering and medical approaches (e.g., cell therapy).
- adipose-derived stem cells
- biomaterials
- stiffness
1. Introduction
Cells continuously interact at a high level with their surrounding extracellular matrix (ECM) in vivo and growth substrates or scaffolds in vitro and modulate their functional phenotypes to maintain homeostasis [1][2][3]. The surrounding material can provide cues that control migration, adhesion, proliferation, and even differentiation of the cells. These cues include physicochemical properties, such as surface topography, chemical modifications and composition, and mechanical properties. Next to the used biomaterial, the choice of a suitable cell source is crucial for success in tissue engineering approaches. Adipose-derived stem cells (ASC), a subpopulation of mesenchymal stem cells (MSC), represent a promising cell source regarding several tissue engineering aims. In contrast to the widely used bone marrow-derived MSCs, they can be isolated in higher numbers with less invasive techniques and can be differentiated into different cell-lineages, which are in strong demand for tissue engineering approaches [4][5]. In the context of cell-based therapies and tissue engineering, it is essential to understand how the physicochemical properties of material surfaces influence ASC behavior. In the last years, a great effort was made to investigate the interactions on the cell–material interface. Understanding these interactions would be a milestone in medical engineering and regenerative medicine by providing specific material designs, which would facilitate and promote tissue repair and regeneration. For tissue engineering, the challenge is to create an in vitro matrix, which promotes progenitor cell migration, adhesion, and proliferation and induces differentiation, extracellular matrix synthesis, and integration with host tissue. Therefore, the major approaches in developing new biomaterials are mimicking certain advantageous characteristics of the natural ECM of the specific cells.
Material properties are an important tool to influence adipose-derived stem cells (ASCs)’ behavior and fate. Table 1 gives an overview of the specific characteristics that can be identified to induce or enhance adipogenic, chondrogenic, or osteogenic differentiation. The most consistent results were found for material stiffness. As tissue stiffness changes during development, it would be interesting to investigate the influence of adjustable stiffness over the culture period.
Table 1. Overview of material characteristics that support adipogenic, chondrogenic, and osteogenic differentiation of ASCs.
| Differentiation | Material Characteristics |
|---|---|
| Adipogenic | Softer materials (comparable to native tissue), lager pores that allow rounded shape and lipid storage, surface functionalization with methyl groups adipose tissue-derived and pre-adipocyte-derived ECM. |
| Chondrogenic | Material stiffness in the medium range, topography that allows the spheroid formation and chondrocyte imprint, surface functionalization with carboxy groups chondrocyte-derived ECM. |
| Osteogenic | Stiff materials, smaller pores, aligned fiber/grooves and nodular or pillar structures and osteoblast imprint, surface functionalization with amine groups or strontium bone tissue-derived and pre-osteoblast-derived ECM. |
2. Adipose-Derived Stem Cells
MSCs are multipotent stem cells that can differentiate into multiple cell types of the mesoderm, such as chondrocytes, osteoblasts, and adipocytes, and non-mesenchymal cell lines, such as neuronal cells, cardiac cells, and skeletal muscle cells. MSCs can be isolated from a wide range of tissues including bone marrow, umbilical cord stroma, and adipose tissue [6]. They exhibit a spindle-shaped fibroblast-like morphology. According to the Minimal Criteria of the International Society of Cellular Therapy, MSCs have to be plastic adhered, exhibit tri-lineage differentiation potential (adipogenic, osteogenic, and chondrogenic), and express cluster of differentiation (CD)73, CD90 and CD105 [7].
ASCs are part of the stromal vascular fraction (SVF) of adipose tissue from where they can be isolated by enzymatic digestion [8]. The SVF of a tissue describes the entirety of all cells of blood vessels and stroma [9][10]. Despite defining ASCs is still a challenge, CD13, CD29, and CD44 count as the unofficial markers for ASCs [11]. Compared to widely used bone marrow-derived MSCs, ASCs have several advantages. For example, they exhibit a 2500-fold higher abundance and a higher differentiation and proliferation potential than bone marrow-derived MSCs. Furthermore, adipose tissue harvest is cheaper, safer, and less invasive compared to bone marrow aspiration [12][13]. However, also ASCs have some limitations regarding tissue harvesting. There is evidence that local anesthetic agents might negatively impact ADSC viability and quantity [14]. Furthermore, liposuction in summer should be avoided due to the risk of infections. Contrary, isolation from whole adipose tissue can be performed every time.
Cultured ASCs secret various immunomodulatory cytokines and growth factors that are relevant for cell therapy [15][16]. It can be suggested that when ASCs are transplanted into inflammatory regions, they actively secret these factors and significantly promote wound healing and tissue repair. This may make ASCs a powerful tool for use in future approaches in the development of cell- and tissue-based therapeutics. To date, there are several clinical trials in several research areas investigating the therapeutic potential of ASCs [17][18][19][20][21][22][23]. However, several points remain unclear. For example, the dependence of differentiation potential from the donor’s age, gender, and anatomic location of the fat source [24]. Despite these limitations, ASCs represent a promising cell source for adipose tissue engineering and regenerative medicine.
Crucial for the determination to differentiate into a particular cell type is the specific differentiation signals received by the cell. These signals can either be soluble factors in the cell culture medium or mechanical signals transmitted through matrix properties. The differentiation process involves a complex and highly orchestrated regulation of the expression of lineage-specific transcription factors (Figure 1). These “master transcriptional regulators” are PPARγ for adipogenic lineage [25], RUNX2 for osteogenic lineage [26], and Sox9 for chondrogenic lineage [27]. Lineage-specific differentiation can be determined by specific proteins. For chondrogenic differentiation, these include the important ECM genes Col2a1 and Acan, Col9a1, Col27a1, and Matn1 [28][29]. Adipogenic differentiation can be proven by proteins involved in insulin sensitivity, lipogenesis, and lipolysis, including fatty acid synthase (FAS), glucose transporter type 4 (GLUT4), lipoprotein lipase (LPL), fatty acid binding protein 4 (FABP4), perilipin, and adipokines [30][31][32][33][34]. Classical osteogenic markers include proteins, such as collagen type I, alkaline phosphatase (ALP), osteocalcin (OCN), and bone sialoprotein (BSP) [35]. Another important transcription factor involved in regulating osteogenic differentiation is the zinc-finger transcription factor osterix (OSX) [36].
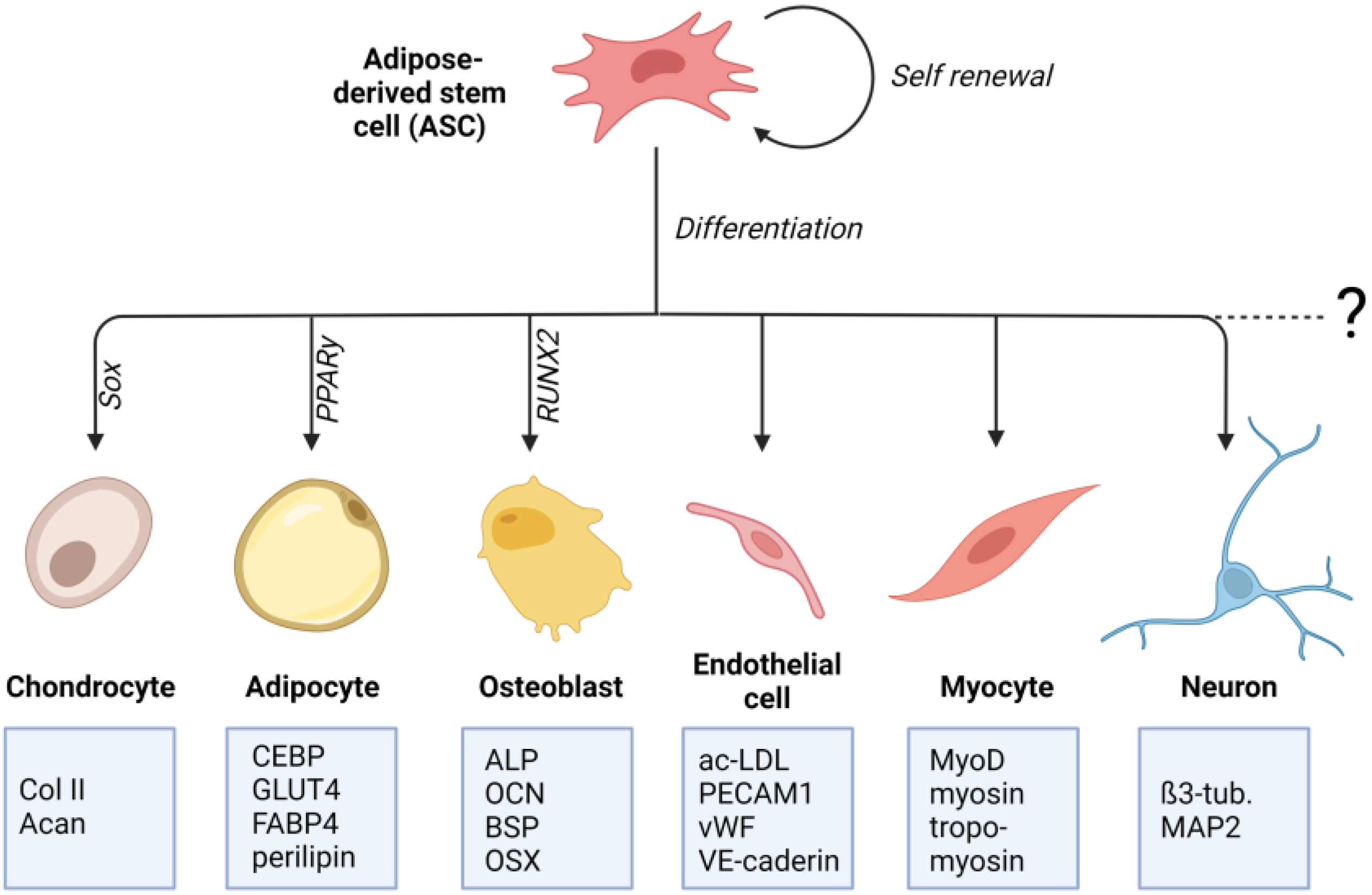
Figure 1. Differentiation potential of adipose-derived stem cells (ASCs): ASCs are multipotent mesenchymal stem cells that can be isolated from adipose tissue. ASCs can differentiate into mesenchymal cell lines such as osteocytes, adipocytes, and chondrocytes. There is also evidence for their differentiating potential into non-mesenchymal cell lines, such as endothelial cells, neuronal cells, and cardiac and skeletal myocytes. For each differentiation lineage, a specific “master transcriptional regulator” is identified. For adipogenic lineage it is PPARγ, for osteogenic lineage it is RUNX2, and for chondrogenic lineage it is Sox9. Lineage-specific differentiation can be determined by specific proteins that are listed in the boxes (created with BioRender.com).
This entry is adapted from the peer-reviewed paper 10.3390/ijms24043551
References
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018.
- Han, S.-B.; Kim, J.-K.; Lee, G.; Kim, D.-H.; Han, S.-B.; Kim, J.-K.; Lee, G.D.; Kim, D.-H. Mechanical Properties of Materials for Stem Cell Differentiation. Adv. Biosyst. 2020, 4, 2000247.
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 283.
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260.
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675.
- Jung, S.; Panchalingam, K.M.; Rosenberg, L.; Behie, L.A. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012, 2012, 123030.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Trojahn Kølle, S.F.; Oliveri, R.S.; Glovinski, P.V.; Kirchhoff, M.; Mathiasen, A.B.; Elberg, J.J.; Andersen, P.S.; Drzewiecki, K.T.; Fischer-Nielsen, A. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy 2013, 15, 1086–1097.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Scioli, M.G.; Bielli, A.; Gentile, P.; Mazzaglia, D.; Cervelli, V.; Orlandi, A. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int. J. Mol. Sci. 2014, 15, 6517–6526.
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492.
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Cartilage, and Adipose Tissue. Stem Cells Dev. 2008, 17, 761–773.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306.
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 2021, 11, 878.
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228.
- Li, P.; Guo, X. A review: Therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration 11 Medical and Health Sciences 1103 Clinical Sciences 10 Technology 1004 Medical Biotechnology. Stem Cell Res. Ther. 2018, 9, 302.
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemotherapy 2016, 43, 268–274.
- Alió Del Barrio, J.L.; El Zarif, M.; De Miguel, M.P.; Azaar, A.; Makdissy, N.; Harb, W.; El Achkar, I.; Arnalich-Montiel, F.; Alió, J.L. Cellular Therapy with Human Autologous Adipose-Derived Adult Stem Cells for Advanced Keratoconus. Cornea 2017, 36, 952–960.
- Jurado, M.; De La Mata, C.; Ruiz-García, A.; López-Fernández, E.; Espinosa, O.; Remigia, M.J.; Moratalla, L.; Goterris, R.; García-Martín, P.; Ruiz-Cabello, F.; et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy 2017, 19, 927–936.
- Pourmand, G.; Arjmand, B.; Safavi, M.; Heidari, R.; Aghayan, H.; Bazargani, S.T.; Dehghani, S.; Goodarzi, P.; Mohammadi-Jahani, F.; Heidari, F.; et al. Concomitant Transurethral and Transvaginal-Periurethral Injection of Autologous Adipose Derived Stem Cells for Treatment of Female Stress Urinary Incontinence: A Phase One Clinical Trial. Acta Med. Iran. 2017, 19, 368–374.
- Sarveazad, A.; Newstead, G.L.; Mirzaei, R.; Joghataei, M.T.; Bakhtiari, M.; Babahajian, A.; Mahjoubi, B. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: Preliminary findings of a randomized double-blind clinical trial. Stem Cell Res. Ther. 2017, 8, 40.
- Tsai, Y.A.; Liu, R.S.; Lirng, J.F.; Yang, B.H.; Chang, C.H.; Wang, Y.C.; Wu, Y.S.; Ho, J.H.C.; Lee, O.K.; Soong, B.W. Treatment of Spinocerebellar Ataxia With Mesenchymal Stem Cells: A Phase I/IIa Clinical Study. Cell Transplant. 2017, 26, 503–512.
- Bailey, A.M.; Kapur, S.; Katz, A.J. Characterization of Adipose-Derived Stem Cells: An Update. Curr. Stem Cell Res. Ther. 2010, 5, 95–102.
- Farmer, S.R. Regulation of PPARγ activity during adipogenesis. Int. J. Obes. 2005, 29 (Suppl. 1), S13–S16.
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286.
- Yi, S.W.; Kim, H.J.; Oh, H.J.; Shin, H.; Lee, J.S.; Park, J.S.; Park, K.H. Gene expression profiling of chondrogenic differentiation by dexamethasone-conjugated polyethyleneimine with SOX trio genes in stem cells. Stem Cell Res. Ther. 2018, 9, 341.
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; De Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828.
- Nishimura, R.; Hata, K.; Ikeda, F.; Ichida, F.; Shimoyama, A.; Matsubara, T.; Wada, M.; Amano, K.; Yoneda, T. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J. Bone Miner. Metab. 2008, 26, 203–212.
- Frith, J.; Genever, P. Transcriptional control of mesenchymal stem cell differentiation. Transfus. Med. Hemother. 2008, 35, 216–227.
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S.; et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952.
- Nielsen, R.; Pedersen, T.Å.; Hagenbeek, D.; Moulos, P.; Siersbæk, R.; Megens, E.; Denissov, S.; Børgesen, M.; Francoijs, K.J.; Mandrup, S.; et al. Genome-wide profiling of PPARγ:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008, 22, 2953–2967.
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617.
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158.
- Li, Y.; Ge, C.; Long, J.P.; Begun, D.L.; Rodriguez, J.A.; Goldstein, S.A.; Franceschi, R.T. Biomechanical Stimulation of Osteoblast Gene Expression Requires Phosphorylation of the RUNX2 Transcription Factor. J. Bone Miner. Res. 2012, 27, 1263–1274.
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29.
This entry is offline, you can click here to edit this entry!
