The entry of peptides into glycobiology has led to the development of a unique class of therapeutic tools. Although numerous and well-known peptides are active as endocrine regulatory factors that bind to specific receptors, and peptides have been used extensively as epitopes for vaccine production, the use of peptides that mimic sugars as ligands of lectin-type receptors has opened a unique approach to modulate activity of immune cells. Ground-breaking work that initiated the use of peptides as tools for therapy identified sugar mimetics by screening phage display libraries. The peptides that have been discovered show significant potential as high-avidity, therapeutic tools when synthesized as multivalent structures.
- peptides
- eczema
- transglutaminase 2
1. Introduction
The extracellular domain of most proteins in the cell membrane is extensively decorated with carbohydrate groups. The plethora of glycans is matched by the vast diversity of lectin-type receptors. Single glycans bind with low affinity to these receptors but occur at a density sufficient to maintain physiological homeostasis. Nevertheless, to modulate an immune response under this canopy of sugars requires high-affinity ligands to activate or inhibit cellular processes. Modulators of the immune system with high activity often have a sense of multivalency, if not actually structurally multivalent. The chemistry of synthesis of multivalent glycan structures has advanced rapidly over the past few years [1][2][3][4][5][6][7]. However, glycans generally have poor drug-like properties. These concerns led to extensive chemical modifications of sugars and sugar-like structures that can serve as “glycomimetic” drugs [8][9]. Yet, it is useful to synthesize peptide mimetics of the native glycan ligand, which provide a relatively high specificity along with a high avidity of binding to receptors [10][11]. The sequences of glycomimetic peptides have usually been discovered by screening phage display libraries with lectins, a highly effective method of identifying peptides that bind to sugar-binding sites [12][13][14][15][16][17][18].
Protocols for screening libraries were described by Matsubara [12] and Yu et al. [13], who remarked that “these sequences would never have been predicted rationally nor would molecules with similar modes of binding have [otherwise] been discovered.” Structures larger than tetravalent are often antigenic, and an active area of research has been the search for peptide vaccines that induce the immune system to produce antibodies against viral or tumor-associated carbohydrate structures [19]. Whereas a vaccine initiates the pathway in T cells but is not the target to which the final antibodies bind, in other uses the multivalent glycomimetic peptides are direct ligands for receptors [20][21][22].
2. Peptides in Treatment of Neutrophilic Skin Diseases
Central to the homeostasis of the skin in the mouse are two related receptors, the Gal-specific CD301a (MGL1) and the GalNAc-specific CD301b (MGL2), the ortholog of human CLEC10A, that are expressed by M2a macrophages in the dermis [23][24][25]. A serendipitous but important finding was an additional feature of glycomimetic peptides that is essential in treatment of inflammatory skin diseases [25]. Terminal differentiation of keratinocytes in the epidermis yields the stratum corneum, the first line of protection against environmental pathogens and allergens. Disruption of the stratum corneum, either by genetic deficiencies or physical/chemical insults, leads to inflammatory conditions of eczema caused by infiltration of neutrophils into the skin [25][26]. LPS, proteins in house dust mites (HDM), and Staphylococcus enterotoxin B are commonly encountered allergens that cause eczema [27]. CD301a (MGL1) binds Gal residues on the major allergen in dust mites [28], presumably leading to phagocytosis and the destruction of the allergen by macrophages. Kanemaru et al. [29] found that the NC/Nga strain of mouse has a loss-of-function mutation in the gene encoding CD301a (MGL1, Clec10a) and is highly susceptible to LPS- and dust mite-induced infiltration of neutrophils, the primary cause of dermatitis. A high molecular weight glycoprotein that is rich in terminal T (Galβ(1-3)-GalNAcα1-OSer/Thr) and Tn (GalNAcα1-OSer/Thr) antigens was purified from HDM. This glycoprotein inhibited LPS-induced eczema in the tape-stripped skin of wild-type but not of Clec10a-/- mice. This finding indicated that CD301a+ macrophages are a major cell type in the defense against allergens and pathogens. Dupasquier et al. [30][31] found that half of the nucleated cells in the dermis of mouse skin were positive for antibodies against CD301a and CD301b, a characteristic of M2a macrophages. Gene regulatory network modeling of polarization indicated that M2a is the most frequent macrophage phenotype [32]. Efferocytosis (phagocytosis of apoptotic cells) of neutrophils by macrophages [33][34][35][36] is a major event in the resolution of inflammation and restoration of tissue homeostasis.
The HDM allergen [28] induces an inflammatory response through toll-like receptor 4 (TLR4). LPS is a well-documented ligand for TLR4, mediated by CD14, which activates a pathway leading to the transcriptional factor NF-κB. Researchers induced dermatitis on wild-type C57BL/6J mice with a combination of 1% SDS and 10 μg/cm2 LPS and tested whether the peptide mimetics of GalNAc, svL4 and sv6D, would ameliorate neutrophilic-driven eczema similar to that of the glycoprotein described by Kanemaru et al. [29]. Structural disruption of the epidermis was completely repaired within 14 days when 1 μM svL4 was applied topically along with LPS [25]. Restoration of the surface barrier also led to the elimination of neutrophils from the dermis. sv6D was more effective than svL4 when administered subcutaneously, particularly with severely damaged skin as characterized by neutrophilic dermatoses (unpublished results). Because CD301b+ (MGL2+) macrophages are essential for healing wounds in the skin [37][38], the higher avidity of sv6D with the receptor and its smaller molecular size suggest greater access to the dermis and thus promote this peptide as the superior drug for treating eczema and more serious dermatitis diseases.
LPS-induced inflammation leads to a compensatory inhibition by IL-10 [39][40]. IL-10 production is also induced through TLR4 but by a pathway that activates the transcription factors CREB (cAMP response element binding protein) and ATF1 (cAMP-dependent transcription factor 1) [39]. Inhibition of transcription of LPS-stimulated genes is the primary mechanism of IL10-mediated suppression of inflammation in macrophages [41][42]. In mice deficient in the IL-10 receptor, an LPS challenge can be fatal [43]. Relevant to dermatitis, IL-10 is produced by macrophages, DCs, and neutrophils [39][42]. A seminal observation by Zaal et al. [44] was the strong stimulation of IL-10 production in monocyte-derived DCs by a multivalent GalNAc-dendrimer but only in the presence of LPS. Researchers therefore concluded that a possible role of svL4 and sv6D, as mimetics of GalNAc, in rapid restoration of neutrophilic dermatitis was stimulation of IL-10 production by engagement of the receptor CD301b (MGL2) expressed by macrophages in addition to their phagocytic activity that was stimulated by an increase in cytosolic Ca2+ [45].
svL4 contains glutamine residues that are an α-helical turn distant from each other and is a functional substrate of transglutaminase 2 (TGM2), also called tissue transglutaminase. Peptide sv6D, the C-terminal half of svL4, contains a glutamine residue at position 2 and is also a substrate for TGM2 [25]. Transglutaminases are critically important for functional development of the skin, and TGM2 and other isoforms are strongly induced by inflammatory conditions when the skin is damaged by wounds [46][47][48] or disease [49][50]. TGM2 catalyzes the formation of ε(γ-glutamyl)lysine iso-peptide bonds between glutamine and lysine residues, but the paucity of available glutamine residues at the skin surface seems to limit the ability of the enzyme to tighten the surface barrier and prevent transepithelial loss of water. Thus, healing occurs slowly. The topical application of the peptides provided abundant glutamine residues as substrates for cross-linking reactions. In this mouse model of eczema, the peptide rapidly restores the epidermis to normal morphology and promotes the elimination of the drivers of inflammation, neutrophils, from the dermis.
TGM2 occurs within cells that express the enzyme but is also secreted by a non-classical mechanism [51]. In the cytosol, TGM2 exists in an inactive “closed” conformation in which the catalytic site with the reactive cysteine residue is covered by domains β-barrel1 and β-barrel2, which hinders access of the substrate. The closed conformation is stabilized by GTP or GDP bound to β-barrel1 (within the circle in Figure 1). In the extracellular environment, TGM2 undergoes a dramatic conformational change, from the closed form to a fully ‘open’ form in which Ca2+ displaces the guanine nucleotide and stabilizes the active enzyme [52]. The open form of the enzyme requires millimolar concentrations of Ca2+ for activity [53]. As shown in Figure 1, bound to the catalytic site is an inhibitory peptide, a derivative of a substrate, Ac-PQLPF-NH2, in which the glutamine is replaced with 6-diazo-5-oxo-L-norleucine [52], that stabilizes the open form. The enzyme freshly dissolved from a lyophilized protein was active in an assay with svL4, the tetravalent peptide with 12-mer active arms plus a 4-mer linker to the tri-lysine core, but not with sv6D, which has an active 6-mer sequence. The Km with svL4 as substrate was approximately 7 μM. However, after the solution was stored for 3 days at 5 °C, sv6D and svL4 were equally active. Possibly, the longer arm was able to gain access to the active site cysteine but access by the shorter arm was restricted by the remainder of the tetravalent structure. These results suggested that the enzyme structure slowly gained the open conformation with which access to the active site was less hindered. In a condition such as eczema, which persists for extended periods of time, TGM2 will likely exist as newly synthesized (closed) and aged (open) forms. Thus, a treatment with svL4 or sv6D should essentially be equally effective.
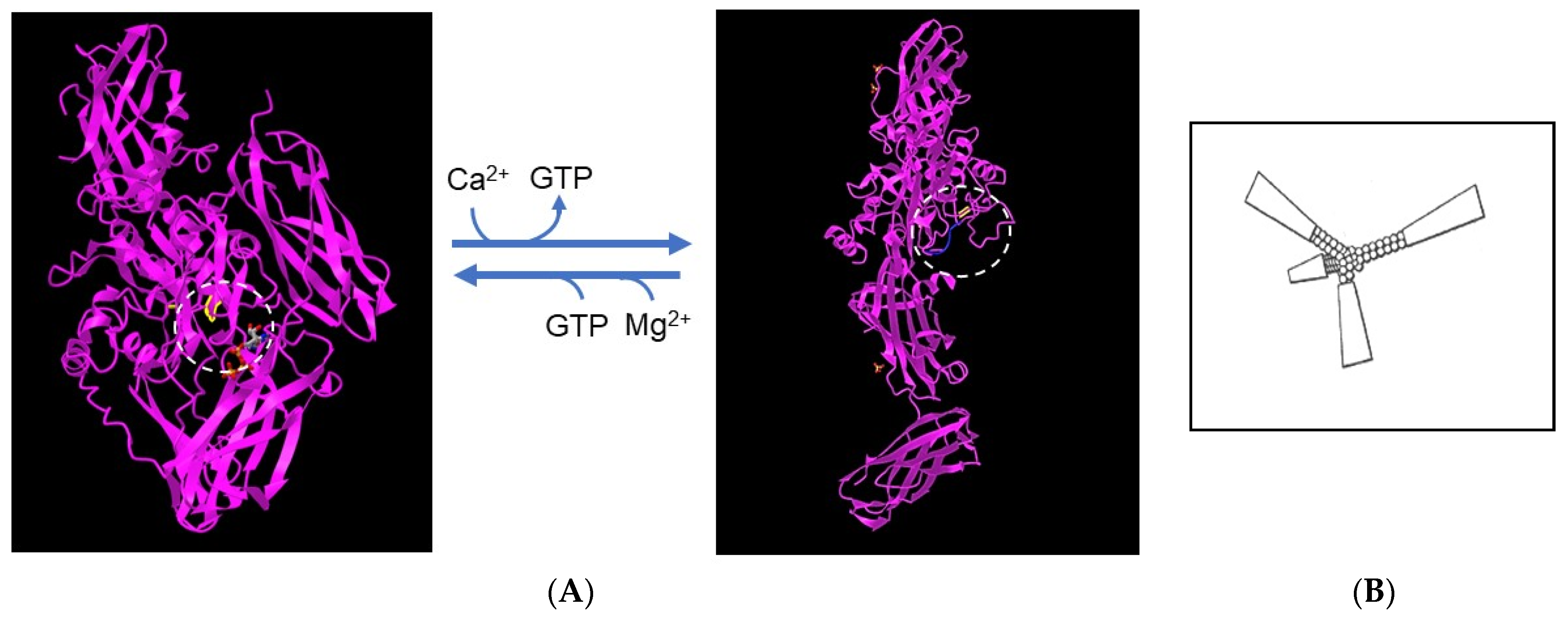
Figure 1. Structures of transglutaminase 2. (A) The inactive, closed conformation (accession no. 3LY6) (left) and the fully open, active conformation (accession no. 2Q3Z) (right). The catalytic site of the enzyme is circled and the position of the cysteine residue that forms the thioester linkage to the substrate in the first step in the reaction is highlighted. The closed conformation is stabilized by Mg2+ and a GTP molecule, shown in gray, that binds to β-barrel1 and covers the catalytic site. Calcium displaces Mg2+ and GTP and generates the open conformation, which is stabilized by an inactive derivative of the peptide substrate. As shown within the box at the right, the remainder of the tetravalent structure of svL4 and sv6D may restrict entry of an arm into the catalytic site of the closed or partially open conformation. (B) Drawing of the tetravalent peptide structure.
In addition to the essential process of repair of the surface barrier, activation of dermal macrophages may also contribute to healing of the injury. As described by Shook et al. [37], macrophages that express MGL2 (CD301b) are essential for wound healing. With healthy skin, it is generally considered that molecules larger than 500 Da cannot penetrate the stratum corneum [54]. Formulation protocols for larger drugs and biomolecules have been developed for transdermal delivery [55][56]. However, eczema and neutrophilic dermatoses are characterized by a loose surface barrier, disruption of the epidermis, or even complete loss of the epithelium. In these cases, svL4 or sv6D could diffuse into the dermal regions of the skin, promote a reduction in neutrophils and inflammation as well as subsequent healing by engaging dermal macrophages. The peptides therefore appear to be excellent tools for the repair of damaged skin by a two-pronged mechanism, as a cross-linking substrate for TGM2 to repair the surface barrier and as a ligand of CD301b (CLEC10A) for activation of dermal macrophages to eliminate neutrophils.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020688
References
- Khorev, O.; Stokmaier, D.; Schwardt, O.; Cutting, B.; Ernst, B. Trivalent, Gal/GalNAc-containing ligands designed for asialoglycoprotein receptor. Bioorg. Med. Chem. 2008, 16, 5216–5231.
- Mūller, C.; Despras, G.; Lindhorst, T.K. Organizing multivalency in carbohydrate recognition. Chem. Soc. Rev. 2016, 45, 3275–3302.
- Mende, M.; Tsouka, A.; Heidepriem, J.; Paris, G.; Mattes, D.S.; Eickelmann, S.; Bordoni, V.; Wawrzinek, R.; Fuchsberger, F.F.; Seeberger, P.H. On-chip neo-glycopeptide synthesis for multivalent glycan presentation. Chem. Eur. J. 2020, 26, 9954–9963.
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’In, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961.
- Prakash, T.P.; Yu, J.; Migawa, M.T.; Kinberger, G.A.; Wan, W.B.; Østergaard, M.E.; Vasquez, G.; Low, A.; Chappell, A.; Schmidt, K.; et al. Comprehensive structure-activity relationship of triantennary N-acetylgalactosamine conjugated antisense oligonucleotides for targeted delivery to hepatocytes. J. Med. Chem. 2016, 59, 2718–2733.
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; de Gruijl, T.D.; Garcia-Vallejo, J.J.; van Kooyk, Y. Glyco-dendrimers as intradermal anti-tumor vaccine targeting multiple skin DC subsets. Theranostics 2019, 9, 5797–5809.
- Gao, T.; Yan, J.; Liu, C.-C.; Palma, A.S.; Guo, Z.; Xiao, M.; Chen, X.; Liang, X.; Chai, W.; Cao, H. Chemoenzymatic synthesis of O-mannose glycans containing sulfated or nonsulfated HNK-1 epitope. J. Am. Chem. Soc. 2019, 141, 19351–19359.
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677.
- Hevey, R. Strategies for the development of glycomimetic drug candidates. Pharmaceuticsls 2019, 12, 55.
- Johnson, M.A.; Pinto, B.M. Structural and functional studies of peptide-carbohydrate mimicry. Top. Curr. Chem. 2008, 273, 55–116.
- Agostino, M.; Sandrin, M.; Thompson, P.; Farrugia, W.; Ramsland, P.; Yuriev, E. Carbohydrate-mimetic peptides: Structural aspects of mimicry and therapeutic implications. Exp. Op. Biol. Ther. 2011, 11, 211–224.
- Matsubara, T. Potential of peptides as inhibitors and mimotopes: Selection of carbohydrate-mimetic peptides from phage display libraries. J. Nuleic Acids 2012, 2012, 740982.
- Yu, L.; Yu, P.S.; Mui, E.Y.Y.; McKelvie, J.C.; Pham, T.P.T.; Yap, Y.W.; Wong, W.Q.; Wu, J.; Deng, W.; Orner, B.P. Phage display screening against a set of targets to establish peptide-based sugar mimetics and molecular docking to predict binding site. Bioorg. Med. Chem. 2009, 17, 4825–4832.
- Scott, J.K.; Smith, G.P. Searching for peptide ligands with an epitope library. Science 1990, 249, 386–390.
- Devlin, J.J.; Panganiban, L.C.; Devlin, P.E. Random peptide libraries: A souce of specific protein binding molecules. Science 1990, 249, 404–406.
- Bächle, D.; Loers, G.; Guthöhrlein, E.W.; Schachner, M.; Sewald, N. Glycomimetic cyclic peptides stimulate neurite outgrowth. Angew. Chem. Int. Ed. 2006, 45, 6582–6585.
- Bhunia, A.; Vivekanandan, S.; Eckert, T.; Bung-Roderfeld, M.; Wechselberger, R.; Romanuka, J.; Bächle, D.; Kornilov, A.V.; von der Lieth, C.-W.; Jiménez-Barbero, J.; et al. Why structurally different cyclic peptides can be glycomimetics of the HNK-1 carbohydrate antigen. J. Am. Chem. Soc. 2010, 132, 96–105.
- Pashov, A.D.; Plaxco, J.; Kaveri, S.Y.; Monzavi-Karbassi, B.; Harn, D.; Kieber-Emmons, T. Multiple antigenic mimotopes of HIV carbohydrate antigens. Relating structure and antigenicity. J. Biol. Chem. 2006, 281, 29675–29683.
- Kieber-Emmons, T.; Saha, S.; Pashov, A.; Monzavi-Karbassi, B.; Murali, R. Carbohydrate-mimetic peptides for pan anti-tumor responses. Front. Immunol. 2014, 5, 308.
- Eggink, L.L.; Salas, M.; Hanson, C.V.; Hoober, J.K. Peptide sugar mimetics prevent HIV-1 replication in peripheral blood mononuclear cells in the presence of HIV-positive antiserum. AIDS Res. Human Retrovir. 2010, 26, 149–160.
- Eggink, L.L.; Hoober, J.K. Peptide mimetics of terminal sugars of complex glycans. Glycobiol. Insights 2010, 2, 63–74.
- Cote, R.; Eggink, L.L.; Hoober, J.K. CLEC receptors, endocytosis, and calcium signaling. AIMS Aller. Immunol. 2017, 1, 207–231.
- Eggink, L.L.; Roby, K.F.; Cote, R.; Hoober, J.K. An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides. J. ImmunoTher. Cancer 2018, 6, 28.
- Gabba, A.; Bogucka, A.; Luz, J.G.; Diniz, A.; Coelho, H.; Corzana, F.; Cañada, F.J.; Marcelo, F.; Murphy, P.V.; Birrane, G. Crystal structure of the carbohydrate recognition domain of the human macrophage galactose C-type lectin bound to GalNAc and the tumor-associated antigen. Biochemistry 2021, 60, 1327–1336.
- Eggink, L.L.; Hoober, J.K. Resolution of eczema with multivalent peptides. J. Investig. Dermatol. Innovat. 2022, 2, 100142.
- Hoober, J.K.; Eggink, L.L. The discovery and function of filaggrin. Int. J. Mol. Sci. 2022, 23, 1455.
- Kawakami, Y.; Yumoto, K.; Kawakami, T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol. Internatl. 2007, 56, 403–409.
- Johannessen, B.R.; Skov, L.K.; Kastrup, J.S.; Kristensen, O.; Bolwig, C.; Larsen, J.N.; Spangfort, M.; Lund, K.; Gajhede, M. Structure of the house dust mite allergen Der f 2: Implications for function and molecular basis of IgE cross-reactivity. FEBS Lett. 2005, 579, 1208–1212.
- Kanemaru, K.; Noguchi, E.; Tahara-Hanaoka, S.; Mizuno, S.; Tateno, H.; Denda-Nagai, K.; Fujisawa, Y.; Nakamura, Y.; Irimura, T.; Matsuda, H.; et al. Clec10a regulates mite-induced dermatitis. Sci. Immunol. 2019, 4, eaax6908.
- Dupasquier, M.; Stoitzner, P.; van Oudenaren, A.; Romani, N.; Leenen, P.J.M. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J. Investig. Dermatol. 2004, 123, 876–879.
- Dupasquier, M.; Stoitzner, P.; Wan, H.; Cerqueira, D.; van Oudenaren, A.; Voerman, J.S.A.; Denda-Nagai, K.; Irimura, T.; Raes, G.; Romani, N.; et al. The dermal microenvironment induces the expression of the alternate activation marker CD301/mMGL in mononuclear phagocytes, independent of IL-4/IL-13 signaling. J. Leukoc. Biol. 2006, 8, 838–849.
- Palma, A.; Jarrah, A.S.; Tieri, P.; Cesareni, G.; Castiglione, F. Gene regulatory network modeling of macrophage differentiation corroborates the continuum hypothesis of polarization states. Front. Physiol. 2018, 9, 1659.
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995.
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370.
- Zhong, X.; Lee, H.-N.; Kim, S.H.; Park, S.-A.; Kim, W.; Cha, Y.-N.; Surh, Y.-J. Myc-nick promotes efferocytosis through M2 macrophage polarization during resolution of inflammation. FEBS J. 2018, 32, 5312–5325.
- Filep, J.G. Targeting neutrophils for promoting the resolution of inflammation. Front. Immunol. 2022, 13, 866747.
- Shook, B.; Xiao, E.; Kumamoto, Y.; Iwasaki, A.; Horsley, V. CD301b+ macrophages are essential for effective skin wound healing. J. Investig. Dermatol. 2016, 136, 1885–1891.
- Shook, B.A.; Wasko, R.R.; Rivera-Gonzalez, G.C.; Salazar-Gatzimas, E.; López-Giráldez, F.; Dash, B.C.; Muñoz-Rojas, A.R.; Aultman, K.D.; Zwick, R.K.; Lei, V.; et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362, 909.
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2019, 217, 0418.
- Adib, Y.; Bensussan, A.; Michel, L. Cutaneous wound healing: A review about innate immune response and current therapeutic applications. Med. Inflamm. 2022, 2022, 5344085.
- Conaway, E.A.; de Oliveira, D.C.; McInnis, C.M.; Snapper, S.B.; Horwitz, B.H. Inhibition of inflammatory gene transcription by IL-10 is associated with rapid suppression of LPS-induced enhancer activation. J. Immunol. 2017, 198, 2906–2915.
- Hutchins, A.P.; Takahashi, Y.; Miranda-Saavedra, D. Genomic analysis of LPS-stimulated myeloid cells identifies a common pro-inflammatory response but divergent IL-10 anti-inflammatory responses. Sci. Rep. 2015, 5, 9100.
- Shemer, A.; Scheyltjens, I.; Frumer, G.R.; Kim, J.S.; Grozovski, J.; Ayanaw, S.; Dassa, B.; Van Hove, H.; Chappell-Maor, L.; Boura-Halfon, S.; et al. Interleukin-10 prevents pathological microglia hyperactivation following peripheral endotoxin challenge. Immunity 2020, 53, 1033–1049.
- Zaal, A.; Li, R.J.E.; Lūbbers, J.; Bruijns, S.C.M.; Kalau, H.; van Kooyk, Y.; van Vliet, S.J. Activation of the C-type lectin MGL by terminal GalNAc ligands reduces the glycolytic activity of human dendritic cells. Front. Immunol. 2020, 11, 305.
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68.
- Haroon, Z.A.; Hettasch, J.M.; Lai, T.-S.; Dewhirst, M.W.; Greenberg, C.S. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J. 1999, 13, 1787–1795.
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Natures biological glues. Biochem. J. 2002, 368, 377–396.
- Telei, D.; Griffin, M. Tissue transaminase (TG2)—A wound response enzyme. Front. Biosci. 2006, 11, 867–882.
- Liedén, A.; Winge, M.C.G.; Sääf, A.; Kockum, I.; Ekelund, E.; Rodriguez, E.; Fölster-Holst, R.; Franke, A.; Illig, T.; Tengvall-Linder, M.; et al. Genetic variation in the epidermal transglutaminase genes is not associated with atopic dermatitis. PLoS ONE 2012, 7, e49694.
- Su, H.; Luo, Y.; Sun, J.; Liu, X.; Ling, S.; Xu, B.; Zhang, Y.; Liu, J.; Li, W.; Wang, B.; et al. Transglutaminase 3 promotes skin inflammation in atopic dermatitis by activating monocyte-derived dendritic cells via DC-SIGN. J. Investig. Dermatol. 2020, 140, 170–179.
- Chou, C.-Y.; Streets, A.J.; Watson, P.; Huang, L.; Verderio, E.A.M.; Johnson, T.S. A crucial sequence for transglutaminase type 2 extracellular trafficking in renal tubular epithelial cells lies in its N-terminal β-sandwich domain. J. Biol. Chem. 2011, 286, 27825–27835.
- Pinkas, D.M.; Strop, P.; Brunger, A.T.; Khosla, C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007, 5, e327.
- Folk, J.E.; Mullooly, J.P.; Cole, P.W. Mechanism of action of guinea pig liver transglutaminase. II. The role of metal in enzyme activation. J. Biol. Chem. 1967, 242, 1838–1844.
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169.
- Sano, T.; Okada, A.; Kawasaki, K.; Kume, T.; Fukui, M.; Todo, H.; Sugibayashi, K. Self-assembled structure of α-isostearyl glyceryl ether affects skin permeability—A lamellar with 70-nm spaces and L3 phase enhanced the transdermal delivery of a hydrophilic model drug. AAPS PharmSciTech 2022, 23, 296.
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal delivery systems for biomolecules. J. Pharm. Innovat. 2022, 17, 319–332.
