Several treatment approaches for COVID-19 were employed since the beginning of the pandemic, such as immunomodulatory, antiviral, anti-inflammatory, antimicrobial agents, and again corticosteroids, angiotensin II receptor blockers, and bradykinin B2 receptor antagonists, but many of them were proven ineffective in targeting the virus. So, the identification of drugs to be used effectively for treatment of COVID-19 is strongly needed. Carbazoles represent an interesting class of heterocycles known by their anticancer activity: antibacterial, anti-inflammatory, antifungal, antioxidant, antimicrobial, antiepileptic, antihistamine, antiviral. In addition, numerous carbazole derivatives have also been found to be useful for Alzheimer’s disease.
- COVID-19
- carbazoles
- alkaloids
- Coronaviruses
- SARS-CoV-2
1. SARS-CoV-2 M-Pro Inhibitors
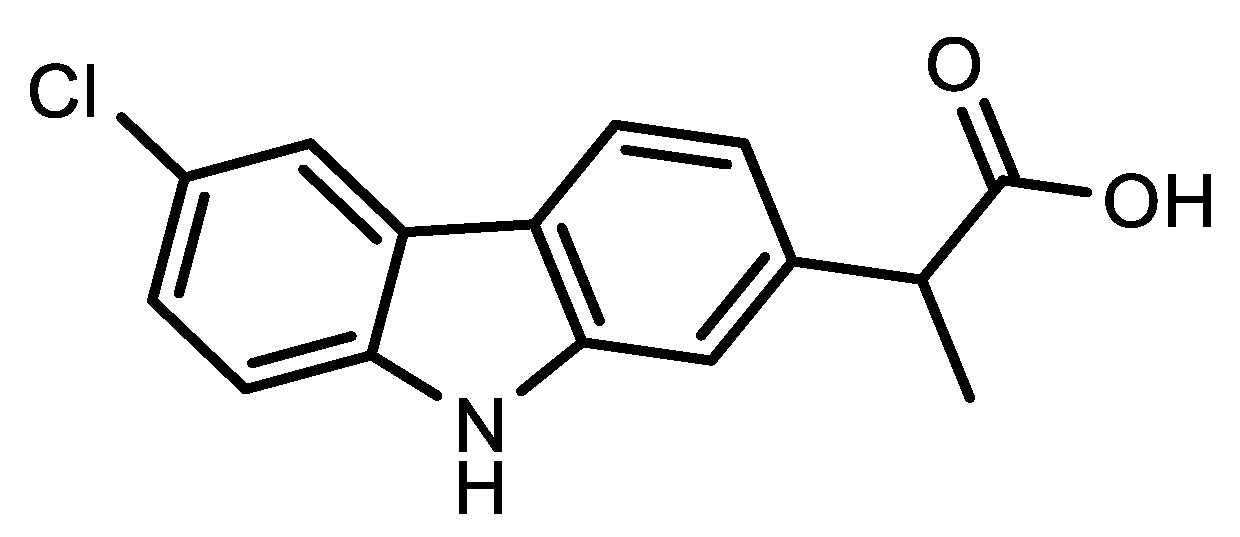
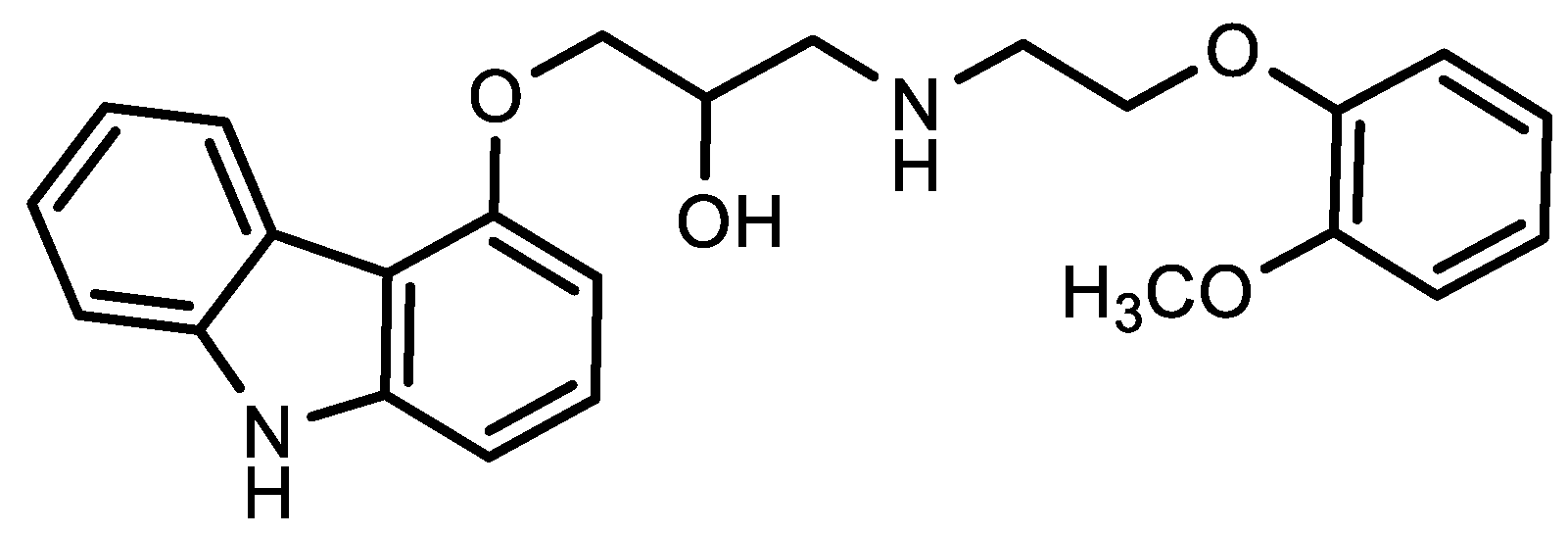
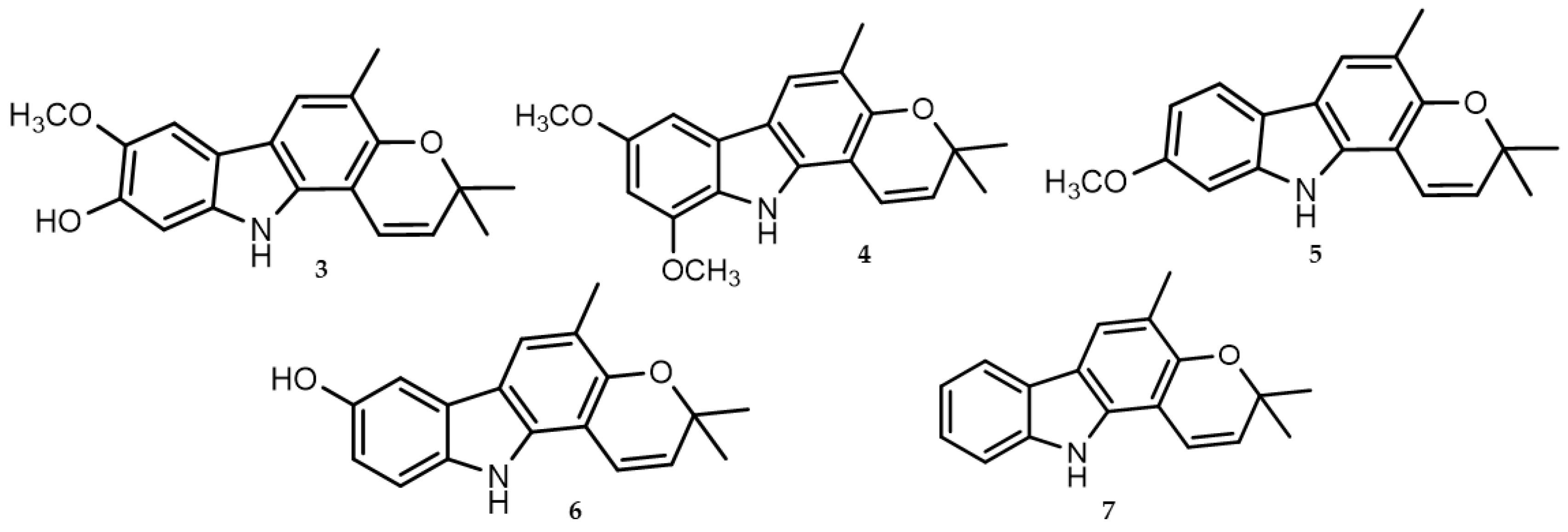
2. Viral-Entry Inhibitors Targeting Human Angiotensin 2 Converting Enzyme Receptor


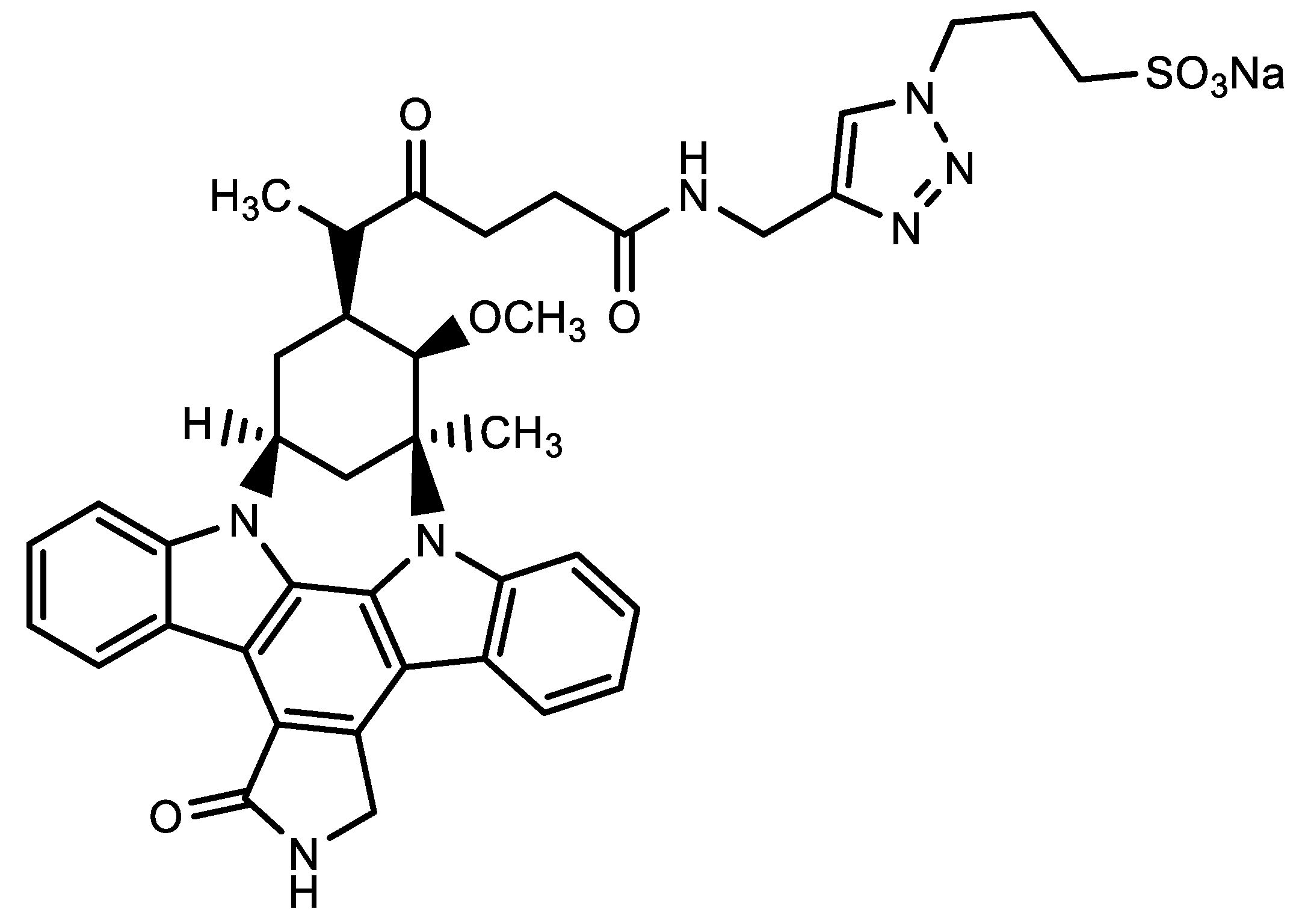
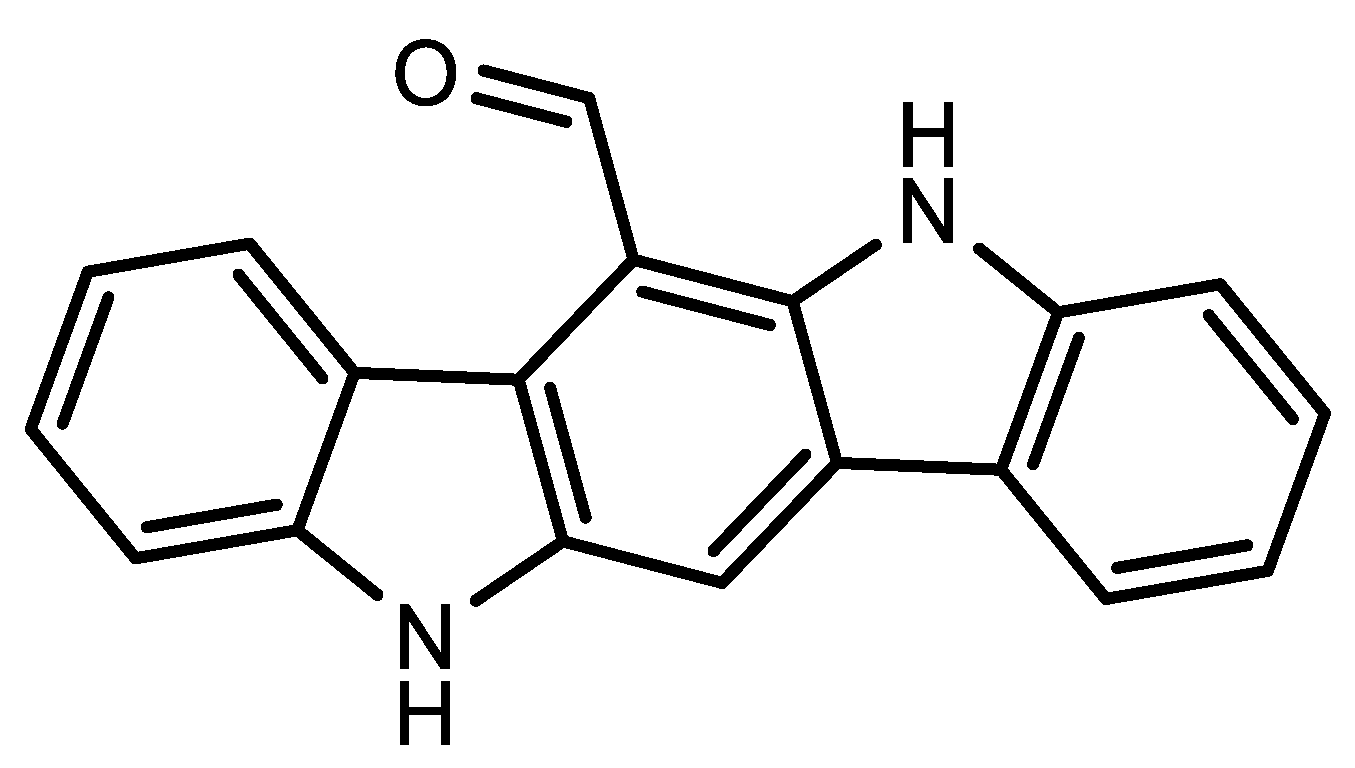
3. Niemann-Pick Type C1 Inhibitor
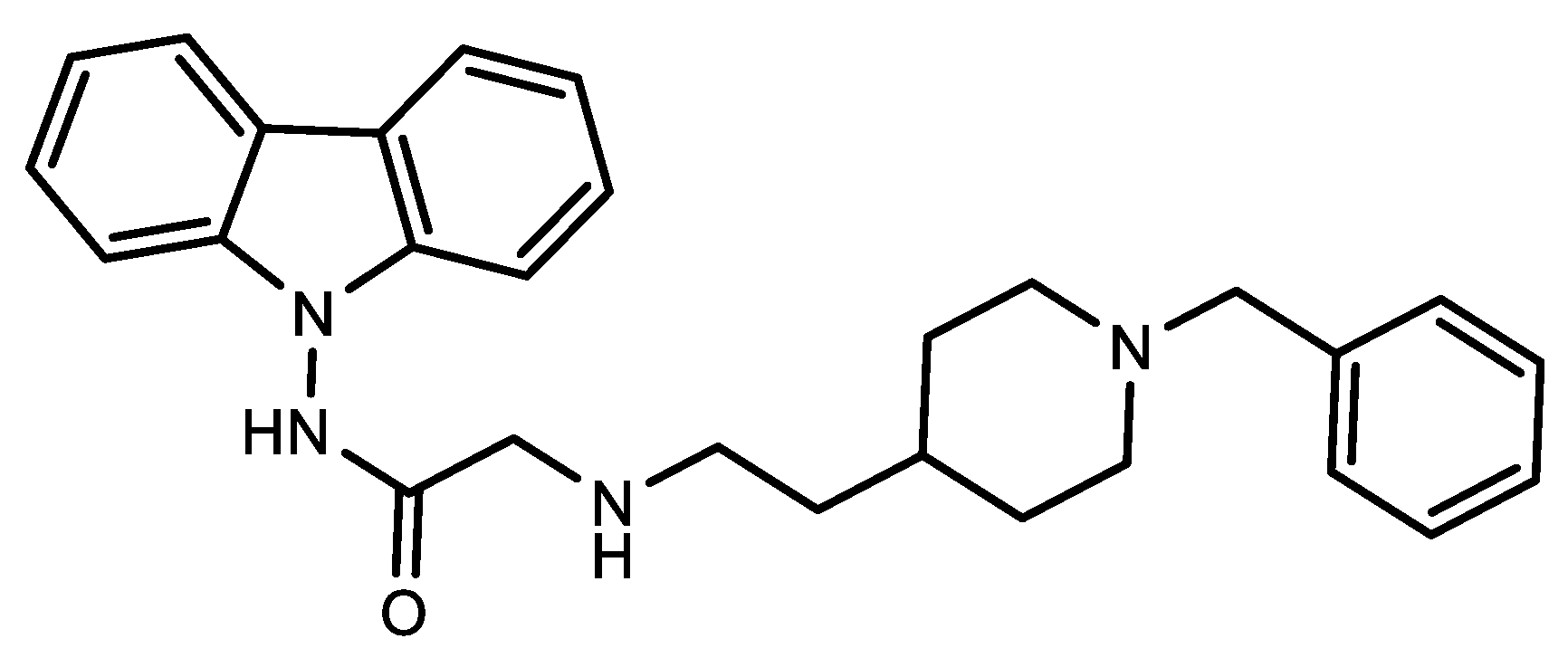
4. Antiviral against Papain-Like Protease
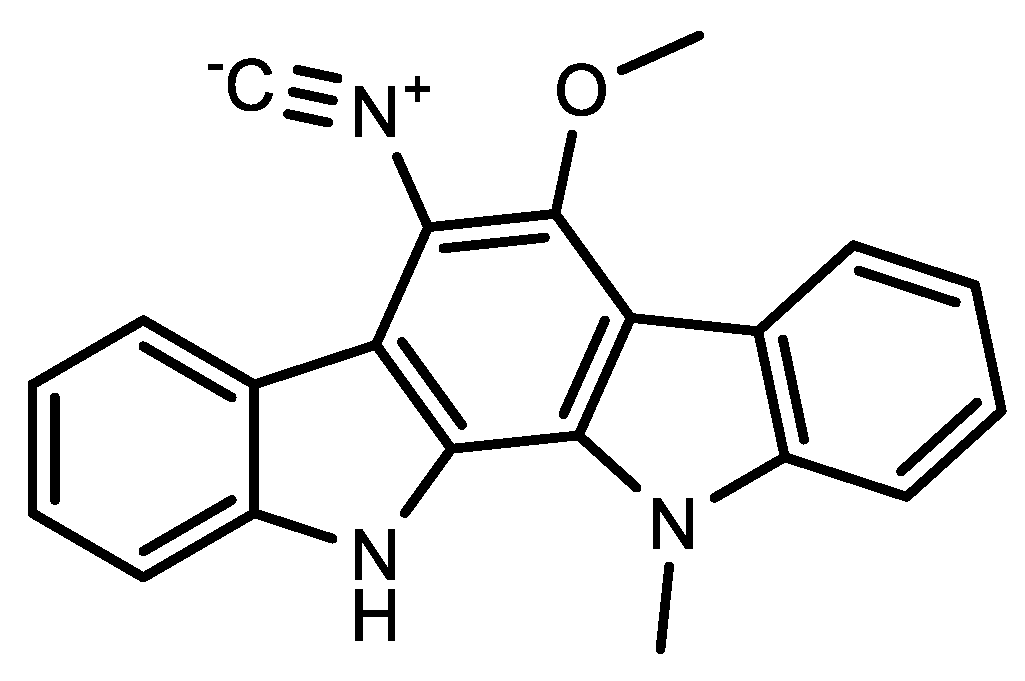
5. Immunotherapy Treatment
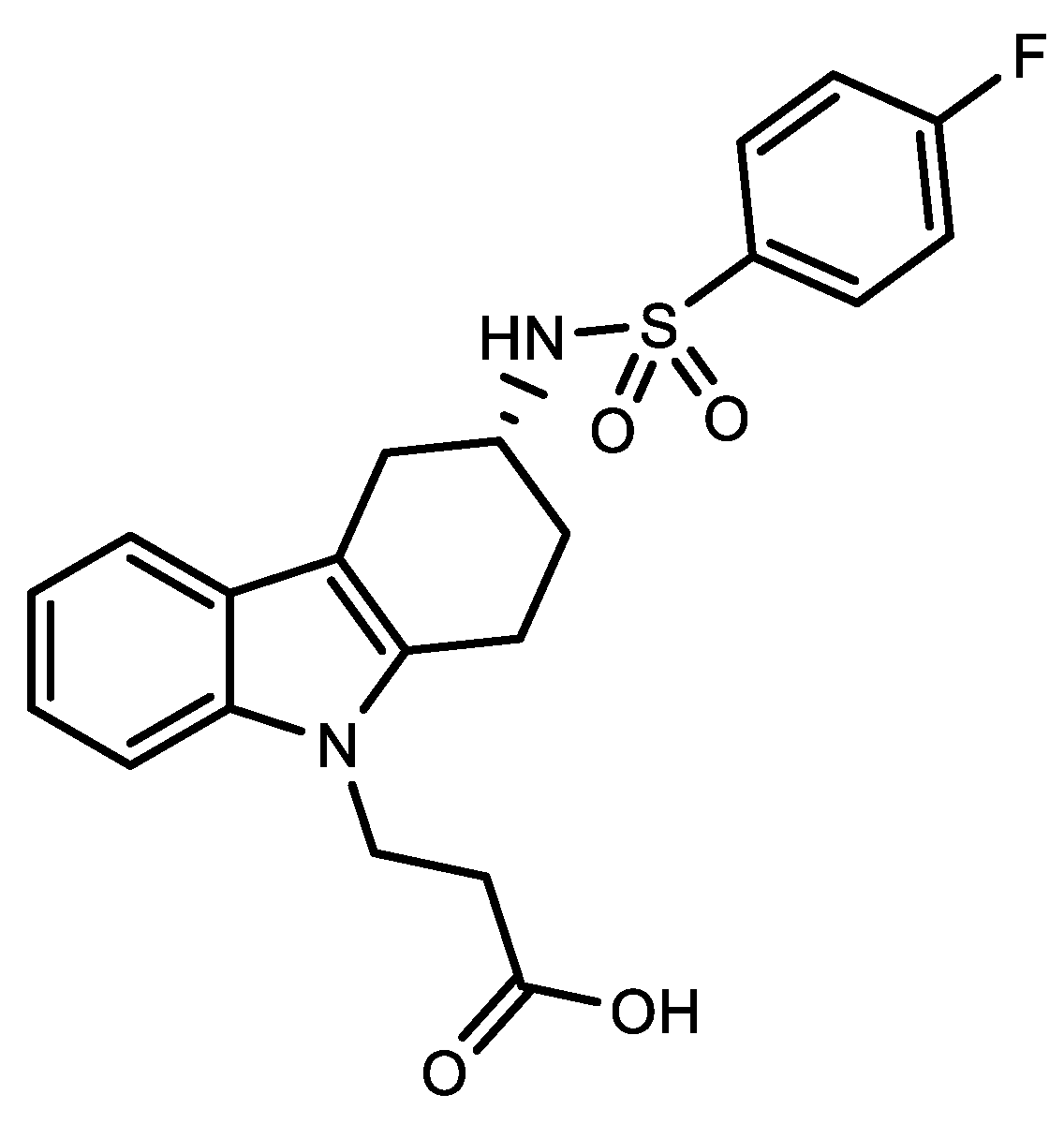
This entry is adapted from the peer-reviewed paper 10.3390/app13031522
References
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412.
- Kong, R.; Yang, G.; Xue, R.; Liu, M.; Wang, F.; Hu, J.; Guo, X.; Chang, S. COVID-19 Docking Server: A meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics 2020, 36, 5109–5111.
- Tang, B.; He, F.; Liu, D.; He, F.; Wu, T.; Fang, M.; Niu, Z.; Wu, Z.; Xu, D. AI-aided design of novel targeted covalent inhibitors against SARS-CoV-2. Biomolecules 2022, 12, 746.
- Gimeno, A.; Mestres-Truyol, J.; Ojeda-Montes, M.J.; Macip, G.; Saldivar-Espinoza, B.; Cereto-Massagué, A.; Pujadas, G.; Garcia-Vallvé, S. Prediction of Novel Inhibitors of the Main Protease (M-pro) of SARS-CoV-2 through Consensus Docking and Drug Reposition. Int. J. Mol. Sci. 2020, 21, 3793.
- Abdel-Halim, H.; Hajar, M.; Hasouneh, L.; Abdelmalek, S.M.A. Identification of Drug Combination Therapies for SARS-CoV-2: A Molecular Dynamics Simulations Approach. Drug Des. Dev. Ther. 2022, 16, 2995–3013.
- Vasanthakumar, N. Beta-Adrenergic Blockers as a Potential Treatment for COVID-19 Patients. BioEssays 2020, 42, 2000094–2000102.
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14.
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of Therapeutic Targets for Sars-Cov-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin. B 2020, 10, 766–788.
- Najmeddin, F.; Solhjoo, M.; Ashraf, H.; Salehi, M.; Rasooli, F.; Ghoghaei, M.; Soleimani, A.; Bahreini, M. Effects of Renin-Angiotensin-Aldosterone Inhibitors on Early Outcomes of Hypertensive COVID-19 Patients: A Randomized Triple-Blind Clinical Trial. Am. J. Hypertens. 2021, 34, 1217–1226.
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681.
- Onohuean, H.; Al Kuraishy, H.M.; Al Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; El Saber Batiha, G. Covid 19 and development of heart failure: Mystery and truth. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 2013–2021.
- Skayem, C.; Ayoub, N. Carvedilol and COVID-19: A potential role in reducing infectivity and infection severity of SARS-CoV-2. Am. J. Med. Sci. 2020, 360, 300.
- Amirshahrokhi, K.; Khalili, A.R. Carvedilol attenuates paraquatinduced lung injury by inhibition of proinfammatory cytokines, chemokine MCP-1, NF-κB activation and oxidative stress mediators. Cytokine 2016, 88, 144–153.
- Congly, S.E.; Sadler, M.D.; Abraldes, J.G.; Tandon, P.; Lee, S.S.; Burak, K.W. Practical management of esophageal varices in the context of SARS-CoV-2 (COVID19): The Alberta protocol. Can. Liver J. 2020, 3, 300–303.
- Jadhav, K.P.; Jariwala, P.V. Ivabradine versus carvedilol in the management of palpitation with sinus tachycardia among recovered COVID-19 patients. J. Cardiol. Cardiovasc. Med. 2020, 1, 10–14.
- Servato, M.L.; Valente, F.X.; García-Moreno, L.G.; Casas, G.; Galera, R.F.; Burcet, G.; Teixidó-Tura, G.; Calabria, H.C.; González, I.F.; Rodríguez-Palomares, J.F. Intraventricular conundrum in a SARS-CoV-2–positive patient with elevated biomarkers of myocardial injury. JACC Case Rep. 2021, 3, 566–572.
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral plant-derived natural products to combat RNA viruses: Targets throughout the viral life cycle. Lett. Appl. Microbiol. 2021, 75, 476–499.
- Balakrishnan, R.; Vijayraja, D.; Jo, S.H.; Ganesan, P.; Su-Kim, I.; Choi, D.K. Medicinal profle, phytochemistry, and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants 2020, 9, 101.
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912.
- Wadanambi, P.M.; Jayathilaka, N.; Seneviratne, K.N. A Computational Study of Carbazole Alkaloids from Murraya koenigii as Potential SARS CoV 2 Main Protease Inhibitors. Appl. Biochem. Biotechnol. 2022, 195, 573–596.
- Schultes, S.; De Graaf, C.; Haaksma, E.E.; De Esch, I.J.; Leurs, R.; Krämer, O. Ligand efciency as a guide in fragment hit selection and optimization. Drug Discov. Today Technol. 2010, 7, e157–e162.
- Guo, Z. The modifcation of natural products for medical use. Acta Pharm. Sin. B. 2017, 7, 119–136.
- Teralı, K.; Baddal, B.; Gülcan, H.O. Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: Insights from a molecular mechanics-assisted structure-based virtual screening experiment. J. Mol. Graph. Model. 2020, 100, 107697–107706.
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: Te major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877.
- Serra, A.; Fratello, M.; Federico, A.; Ojha, R.; Provenzani, R.; Tasnadi, E.; Cattelani, L.; del Giudice, G.; Kinaret, P.A.S.; Saarimäki, L.A.; et al. Computationally prioritized drugs inhibit SARS-CoV-2 infection and syncytia formation. Brief. Bioinform. 2022, 23, bbab507.
- Sampath, D.; Cortes, J.; Estrov, Z.; Du, M.; Shi, Z.; Andreeff, M.; Gandhi, V.; Plunkett, W. Pharmacodynamics of cytarabine alone and in combination with 7- hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood 2006, 107, 2517–2524.
- Santos, F.P.S.; Kantarjian, H.; Cortes, J.; Quintas-Cardama, A. Bafetinib, a dual BcrAbl/Lyn tyrosine kinase inhibitor for the potential treatment of leukemia. Curr. Opin. Investig. Drugs 2010, 11, 1450–1465.
- Cheshenko, N.; Bonanno, J.B.; Hoffmann, H.H.; Jangra, R.K.; Chandran, K.; Rice, C.M.; Almo, S.C.; Herold, B.C. Cell-impermeable staurosporine analog targets extracellular kinases to inhibit HSV and SARS-CoV-2. Commun. Biol. 2022, 5, 1096–1110.
- Tanimoto, K.; Hirota, K.; Fukazawa, T.; Matsuo, Y.; Nomura, T.; Tanuza, N.; Hirohashi, N.; Bono, H.; Sakaguchi, T. Inhibiting SARS CoV 2 infection in vitro by suppressing its receptor, angiotensin converting enzyme 2, via aryl hydrocarbon receptor signal. Sci. Rep. 2021, 11, 16629–16640.
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003.
- García-Dorival, I.; Cuesta-Geijo, M.A.; Barrado-Gil, L.; Galindo, I.; Garaigorta, U.; Urquiza, J.; del Puerto, A.; Campillo, N.E.; Martínez, A.; Gastaminza, P.; et al. Identification of Niemann-Pick C1 protein as a potential novel SARS-CoV-2 intracellular target. Antivir. Res. 2021, 194, 105167–105176.
- Elkaeed, E.B.; Metwaly, A.M.; Alesawy, M.S.; Saleh, A.M.; Alsfouk, A.A.; Eissa, I.H. Discovery of Potential SARS-CoV-2 Papain-like Protease Natural Inhibitors Employing a Multi-Phase In Silico Approach. Life 2022, 12, 1407.
- Gupta, A.; Chiang, K.C. Prostaglandin D2 as a mediator of lymphopenia and a therapeutic target in COVID-19 disease. Med. Hypotheses 2020, 143, 110122–110124.
- Chiang, K.C.; Rizk, J.G.; Nelson, D.J.; Krishnamurti, L.; Subbian, S.; Imig, J.D.; Khan, I.; Reddy, S.T.; Gupta, A. Ramatroban for chemoprophylaxis and treatment of COVID-19: David takes on Goliath. Expert Opin. Ther. Targ. 2022, 26, 13–28.
- Ogletree, M.L.; Kulshreshta, R.; Agarwal, A.; Agarwal, A.; Gupta, A. Treatment of COVID-19 Pneumonia and Acute Respiratory Distress with Ramatroban, a Thromboxane A2 and Prostaglandin D2 Receptor Antagonist: A Four-Patient Case Series Report. Front Pharmacol. 2022, 13, 904020–904037.
