Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hepatocellular carcinoma (HCC) accounts for a great majority of liver cancer diagnoses and deaths. Imaging plays, therefore, a crucial role in the surveillance of patients at risk, the detection and diagnosis of HCC nodules, as well as in the follow-up post-treatment. The unique imaging characteristics of HCC lesions, deriving mainly from the assessment of their vascularity on contrast-enhanced computed tomography (CT), magnetic resonance (MR) or contrast-enhanced ultrasound (CEUS), allow for a more accurate, noninvasive diagnosis and staging.
- hepatocellular carcinoma
- computed tomography
- ultrasound
1. Introduction
Liver malignancies undoubtedly represent a global health challenge, with an estimated annual incidence of more than one million cases in 2025 [1]. Primary liver cancer is the sixth most commonly occurring cancer in the world and the third largest contributor to oncologic mortality [1].
Hepatocellular carcinoma (HCC) accounts for a great majority of liver cancer diagnoses and deaths [2].
Although hepatitis B virus (HBV) and hepatitis C virus (HCV) remain the most important global risk factors worldwide, their impact on the rise of HCC will decline in Western countries due to the availability of increasingly efficient antiviral therapies and preventive policies [3]. As overweight will become endemic worldwide, non-alcoholic fatty liver disease (NAFLD) is likely to become the major contributor to the epidemiology of HCC in the coming years, with a higher risk of incidentally detecting large liver nodules also in younger asymptomatic patients [4]. Other established risk factors of HCC are alcohol consumption [5] and idiopathic liver diseases (e.g., hemochromatosis or primary sclerosing cholangitis) [6].
As a result of several studies on HCC pathology published in the past years, hepatocarcinogenesis is well established nowadays. In cirrhotic livers, metabolic and oxidative insults cause an increased turnover of hepatocytes with a progressive accumulation of genetic mutations [7]. Notably, during the progression from cirrhotic nodules through dysplastic nodules and early HCC to advanced HCC, portal tracts progressively diminish, whereas newly formed unpaired arteries develop due to the tumoral release of vascular endothelial growth factor (VEGF) [7]. Therefore, HCC nodules present a more notable arterial supply as compared to the healthy surrounding parenchyma with the typical greater supply from the portal vein.
Among all the tested serum biomarkers, alpha-fetoprotein (AFP) has proven to improve diagnostic efficiency and to be useful in the evaluation of treatment response in patients with HCC [8].
Unfortunately, the prognosis of patients with HCC remains poor thus far, with an overall ratio of mortality to incidence of 0.91 [9]. However, the accelerated introduction of novel therapeutic modalities is expected to lead to a more favorable scenario. Indeed, due to the recent advances in the oncologic armamentarium, the Barcelona Clinic Liver Cancer (BCLC) treatment strategy was updated in 2022, including the latest evidence of promising medical and interventional therapies [10].
As a matter of fact, in patients at risk, surveillance plays a pivotal role in the detection of small HCC nodules, whose treatment may consist of less invasive and more effective therapies (e.g., percutaneous thermal ablation, surgical excision) [11].
As stated by the latest clinical practice guidelines, published by the European Association for the Study of the Liver (EASL) [12] in 2018, HCC is unique among other cancers in showing typical characteristics on contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) or contrast-enhanced ultrasound (CEUS), thus allowing for a highly accurate diagnosis of HCC in patients with cirrhosis. As a result, mini-invasive percutaneous imaging-guided biopsy is strongly recommended for liver nodules with an atypical contrast enhancement [13] or in non-cirrhotic patients [14].
The ability of cross-sectional imaging studies to reliably detect and diagnose HCC in the cirrhotic liver relies primarily on characterizing the enhancement of a suspected lesion as compared to the background liver parenchyma in the hepatic arterial, portal-venous and subsequent phases. The abovementioned differences in the blood flow and extracellular volume between HCC tissue and non-neoplastic cirrhotic liver tissue result in the hallmark imaging characteristics of HCC during the multiphasic flow of contrast, including arterial phase hyperenhancement, subsequent wash-out appearance and capsule appearance [15].
CEUS is a dynamic imaging technique, able to assess the contrast-enhancement pattern of liver nodules in real time, with a considerably higher temporal resolution than that possible to obtain with CT and MRI [16]. CEUS, however, presents some important drawbacks. First of all, CEUS is not a cross-sectional imaging modality, thus not allowing for the detection of distant nodules not seen or included by the operator in the scan after contrast injection. Moreover, ultrasound (US) examination is an operator-dependent modality and may be limited in the detection of nodules in overweight patients or nodules with a difficult location [17].
MRI offers a number of detailed imaging sequences, including T2-weighted and diffusion-weighted images, which may help in the detection of suspicious nodules, although baseline images rarely provide sufficient specificity to enable noninvasive diagnosis. Furthermore, in recent years, two liver-specific contrast agents (gadobenate dimeglumine and gadoxetic acid) have shown to improve the detection of even relatively small and subtle lesions with a hypointense appearance in the hepatobiliary phase [18].
Nevertheless, MRI has some important diagnostic disadvantages, including less availability, greater technical complexity, higher susceptibility to artifacts, higher costs and less consistent image quality. In particular, MRI quality may be compromised in patients with difficulty in breath-holding, trouble keeping still, or large-volume ascites. MRI permits a locoregional evaluation of parenchyma and nodes in the upper abdomen without any information on possible distant metastases. For these reasons, the comparative diagnostic performance of a multiphasic CT and an MRI in real-life practice remains uncertain [19].
In the recent years a rising interest in artificial intelligence (AI) has been observed, and, undeniably, oncologic imaging is one of the most empowered application fields [20][21][22]. Machine learning (ML) is a branch of AI that focuses on the development of computer algorithms able to learn from structured data to make predictions on decisions without being explicitly programmed to do so. In the oncologic imaging setting, ML is usually combined with radiomics, defined as the process of extracting high-dimensional quantitative features from medical images [23][24][25]. However, radiomic pipeline consists of numerous steps characterized by several factors, leading to a significant variability between studies affecting their repeatability [26][27].
2. Ultrasound
Liver cirrhosis is, thus far, the primary risk factor for HCC, with affected patients requiring periodical imaging surveillance. US is a perfect choice for this purpose due to its safety, wide availability, cost-effectiveness and accuracy in detecting focal liver lesions (FLLs). Once a FLL is detected, US can assist in its characterization using different ultrasonographic techniques, including B-mode, color- and power-Doppler techniques and CEUS [28].
The appearances of HCC nodules on US vary depending on the size and degree of differentiation. The lesion margins are usually relatively well circumscribed in the nodular type but poorly defined in the massive type [29]. HCC nodules smaller than 10 mm are almost hypoechoic or isoechoic, with low-level internal echoes that increase with tissue cellularity. When tumor growth occurs, fatty change is most frequently observed at a tumor diameter of 10–15 mm, and the internal echoes of such nodules are hyperechoic [30]. In HCC nodules greater than 20 mm, typical US patterns such as the “mosaic pattern”, “nodule-in-nodule appearance”, “peripheral sonolucency” (halo sign) and “lateral shadow” can be more commonly recognized [31].
The evaluation of intranodular vascularity may play a key role in the characterization of FLLs. For this purpose, color Doppler is typically the first-line modality of assessment, even though it encounters different technical limitations such as Doppler angle dependence, operator dependence, low sensitivity to slow flow and overwriting artifacts [29]. Usually, once the tumor increases in size, the “basket” pattern, referring to the presence of a fine network of arterial branches surrounding the lesion, can be appreciated [32]. Using spectral analysis, both pulsatile and continuous waveforms can be recorded, which correspond to the arterial and venous origin of blood supply, respectively. In massive-type HCC, an overall irregular pattern of vascularity, can be appreciated. As a general rule, a continuous portal-like waveform indicates a dysplastic nodule or a well-differentiated HCC; contrarily, a pulsatile arterial waveform is suggestive of advanced HCC [29].
Due to the fact that worldwide ultrasound represents the imaging modality of choice in surveilling patients at risk, the introduction of the US LI-RADS® (Liver Imaging Reporting and Data System), a US-based classification system, was issued by the American College of Radiology in 2017 [33]. Evaluating the size and echogenicity, this system assesses the quality of examination and the potential of a FLL to represent HCC and suggests further management [34].
US, in general, has a reported sensitivity of 98% and specificity of 85% for overall HCC detection. Tumor size is nonetheless a significant factor as the technique’s sensitivity reaches approximately 65% for lesions <2 cm [35].
The introduction of CEUS in the evaluation of FLLs certainly represented a turning point in the ultrasonographic diagnosis of HCC. US contrast agents (USCAs) consist of different generations of intravascular gas microbubbles with specific nonlinear acoustic properties [36]. After bolus intravenous injection, USCA allows capillary blood flow to be imaged and contrast enhancement to be assessed, with a much higher temporal resolution compared to CT and MRI [16]. CEUS has proven to be a safe procedure, with low clinical reactions to USCAs reported in the literature and few absolute contraindications (e.g., severe coronary artery disease, pulmonary hypertension). Several studies have stated that CEUS has a significant role as a problem-solving imaging technique for detection of perfusion abnormalities in patients with renal failure and/or at high risk of adverse reaction to CT or MRI contrast agents [17].
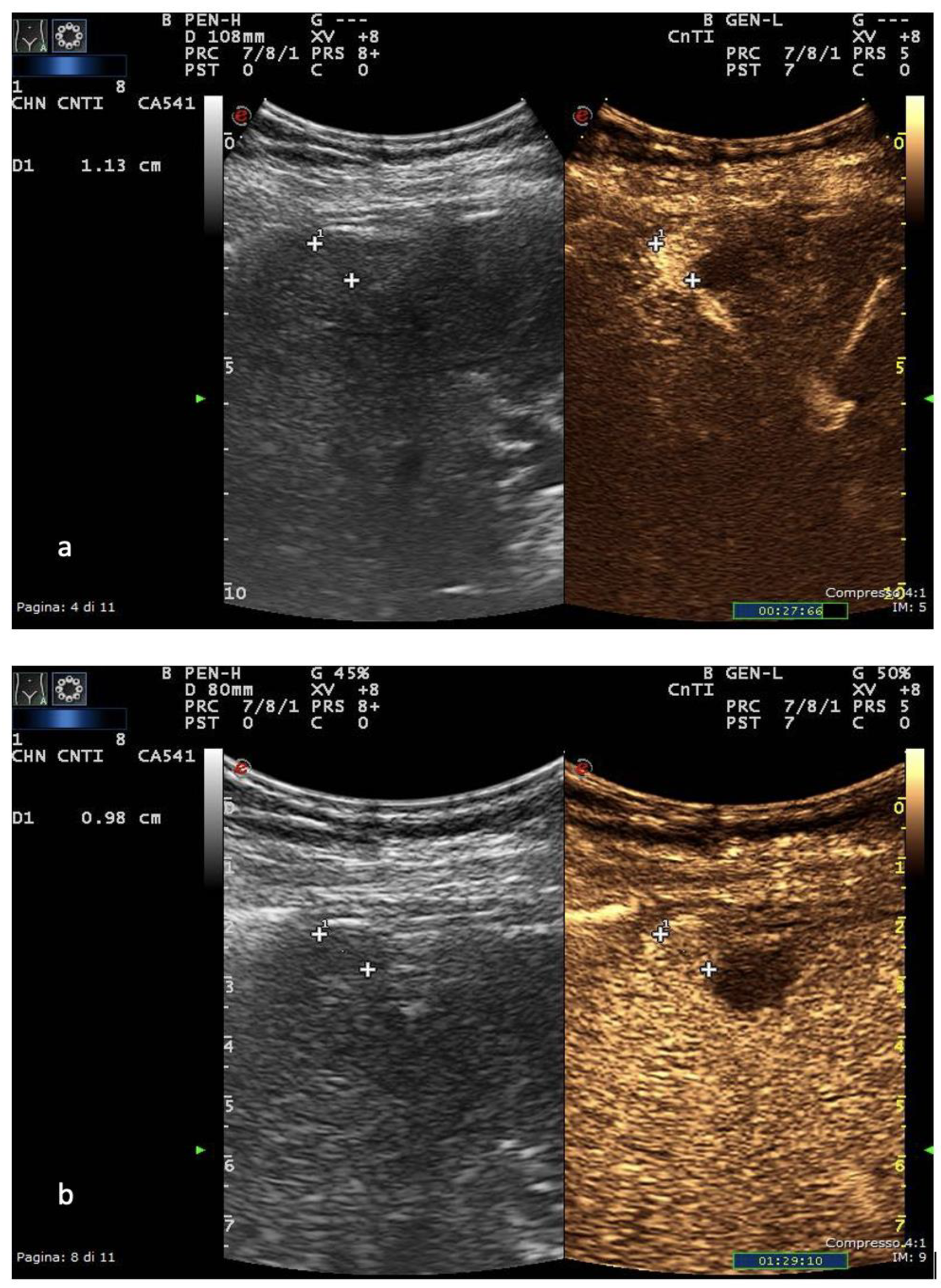
In Europe, CEUS is usually performed with SonoVue® (Bracco, Milan, Italy), which is not uptaken by Kupffer cells and hence produces an arterial, portal-venous and late phase [37]. The hallmark of HCC on CEUS using SonoVue® is a homogeneous and intense arterial phase hyper-enhancement (APHE) with mild wash-out starting >60 s after injection [38] (Figure 1).

Figure 1. US and CEUS surveillance examination in a patient with HBV-related cirrhosis. Baseline images detect the presence of a centimetric subcapsular hypoechoic nodule. After administration of USCA, the lesion shows arterial hyperenhancement (a) with a mild portal-venous wash-out (b).
The timing and degree of wash-out are important for the characterization of HCC, which typically shows milder hypo-enhancement compared to metastasis and cholangiocarcinoma. Nodules measuring >5 cm may show heterogeneous enhancement due to necrosis. Both the size and the degree of differentiation affect the enhancement pattern of HCC [39]. Wash-out is less often seen in HCC nodules <2 cm but is more frequent in HCC with poorer grades of differentiation [40].
On the other hand, Sonazoid® (GE Healthcare, Amersham, UK) is a second-generation USCA whose clinical usage was approved in Japan, South Korea and China. As opposed to Sonovue®, Sonazoid® is uptaken by Kupffer cells and produces a late homonym phase in which HCC nodules appear as hypoechoic lesions as compared to the surrounding parenchyma [41].
Moreover, a CEUS LI-RADS® [42] algorithm has been introduced by the American College of Radiology to aid in the accurate characterization of nodules in liver cirrhosis patients. The major criteria are APHE, nodule size and portal-late mild wash-out. A rim APHE and an early (<60 s) or marked wash-out represent LI-RADS M criteria (LR-M), favoring the diagnosis of a non-hepatocellular malignancy [42].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13040625
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261.
- Alberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; Lesi, O.A.; Hutin, Y.J.-F.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735.
- Onzi, G.; Moretti, F.; Balbinot, S.S.; Balbinot, R.A.; Soldera, J. Hepatocellular carcinoma in non-alcoholic fatty liver disease with and without cirrhosis. Hepatoma Res. 2019, 2019.
- Testino, G.; Leone, S.; Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J. Gastroenterol. 2014, 20, 15943–15954.
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86.
- Takeda, H.; Takai, A.; Eso, Y.; Takahashi, K.; Marusawa, H.; Seno, H. Genetic Landscape of Multistep Hepatocarcinogenesis. Cancers 2022, 14, 568.
- Muscari, F.; Maulat, C. Preoperative alpha-fetoprotein (AFP) in hepatocellular carcinoma (HCC): Is this 50-year biomarker still up-to-date? Transl. Gastroenterol. Hepatol. 2020, 5, 46.
- Markakis, G. The changing epidemiology of hepatocellular carcinoma in Greece. Ann. Gastroenterol. 2022, 35, 88–94.
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2021, 76, 681–693.
- Harris, P.S.; Hansen, R.M.; Gray, M.E.; Massoud, O.I.; McGuire, B.M.; Shoreibah, M.G. Hepatocellular carcinoma surveillance: An evidence-based approach. World J. Gastroenterol. 2019, 25, 1550–1559.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236.
- Russo, F.P.; Imondi, A.; Lynch, E.N.; Farinati, F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig. Liver Dis. 2018, 50, 640–646.
- Desai, A.; Sandhu, S.; Lai, J.-P.; Sandhu, D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019, 11, 1–18.
- Kim, J.H.; Joo, I.; Lee, J.M. Atypical Appearance of Hepatocellular Carcinoma and Its Mimickers: How to Solve Challenging Cases Using Gadoxetic Acid-Enhanced Liver Magnetic Resonance Imaging. Korean J. Radiol. 2019, 20, 1019–1041.
- Eisenbrey, J.R.; Gabriel, H.; Savsani, E.; Lyshchik, A. Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom. Imaging 2021, 46, 3579–3595.
- Bartolotta, T.V.; Taibbi, A.; Midiri, M.; Lagalla, R. Contrast-enhanced ultrasound of hepatocellular carcinoma: Where do we stand? Ultrasonography 2019, 38, 200–214.
- Francisco, F.A.F.; De Araújo, A.L.E.; Neto, J.A.O.; Parente, D.B. Contraste hepatobiliar: Diagnóstico diferencial das lesões hepáticas focais, armadilhas e outras indicações. Radiol. Bras. 2014, 47, 301–309.
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421.
- Romei, C.; Fanni, S.C.; Volpi, F.; Milazzo, A.; D’Amore, C.A.; Colligiani, L.; Neri, E.; De Liperi, A.; Stella, G.M.; Bortolotto, C. New Updates of the Imaging Role in Diagnosis, Staging, and Response Treatment of Malignant Pleural Mesothelioma. Cancers 2021, 13, 4377.
- Chiu, H.-Y.; Chao, H.-S.; Chen, Y.-M. Application of Artificial Intelligence in Lung Cancer. Cancers 2022, 14, 1370.
- Gabelloni, M.; Faggioni, L.; Borgheresi, R.; Restante, G.; Shortrede, J.; Tumminello, L.; Scapicchio, C.; Coppola, F.; Cioni, D.; Gómez-Rico, I.; et al. Bridging gaps between images and data: A systematic update on imaging biobanks. Eur. Radiol. 2022, 32, 3173–3186.
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762.
- Spadarella, G.; Stanzione, A.; D’Antonoli, T.A.; Andreychenko, A.; Fanni, S.C.; Ugga, L.; Kotter, E.; Cuocolo, R. Systematic review of the radiomics quality score applications: An EuSoMII Radiomics Auditing Group Initiative. Eur. Radiol. 2022, 1–11.
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. La Radiol. Medica 2021, 126, 1296–1311.
- Aringhieri, G.; Fanni, S.C.; Febi, M.; Colligiani, L.; Cioni, D.; Neri, E. The Role of Radiomics in Salivary Gland Imaging: A Systematic Review and Radiomics Quality Assessment. Diagnostics 2022, 12, 3002.
- Koçak, B.; Cuocolo, R.; dos Santos, D.P.; Stanzione, A.; Ugga, L. Must-have Qualities of Clinical Research on Artificial Intelligence and Machine Learning. Balk. Med. J. 2023, 40, 3–12.
- Sparchez, Z.; Craciun, R.; Caraiani, C.; Horhat, A.; Nenu, I.; Procopet, B.; Sparchez, M.; Stefanescu, H.; Mocan, T. Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward? J. Clin. Med. 2021, 10, 903.
- Chartampilas, E.; Rafailidis, V.; Georgopoulou, V.; Kalarakis, G.; Hatzidakis, A.; Prassopoulos, P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers 2022, 14, 3997.
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 239–255.
- Minami, Y.; Kudo, M. Hepatic malignancies: Correlation between sonographic findings and pathological features. World J. Radiol. 2010, 2, 249–256.
- Yang, F.; Zhao, J.; Liu, C.; Mao, Y.; Mu, J.; Wei, X.; Jia, J.; Zhang, S.; Xin, X.; Tan, J. Superb microvascular imaging technique in depicting vascularity in focal liver lesions: More hypervascular supply patterns were depicted in hepatocellular carcinoma. Cancer Imaging 2019, 19, 92.
- Ren, A.-H.; Du, J.-B.; Yang, D.-W.; Zhao, P.-F.; Wang, Z.-C.; Yang, Z.-H. The role of ancillary features for diagnosing hepatocellular carcinoma on CT: Based on the Liver Imaging Reporting and Data System version 2017 algorithm. Clin. Radiol. 2020, 75, 478.e25–478.e35.
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830.
- Sangiovanni, A.; Del Ninno, E.; Fasani, P.; De Fazio, C.; Ronchi, G.; Romeo, R.; Morabito, A.; De Franchis, R.; Colombo, M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004, 126, 1005–1014.
- Beckmann, S.; Simanowski, J.H. Update in Contrast-Enhanced Ultrasound. Visc. Med. 2020, 36, 476–486.
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604.
- Fraquelli, M.; Nadarevic, T.; Colli, A.; Manzotti, C.; Giljaca, V.; Miletic, D.; Štimac, D.; Casazza, G. Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst. Rev. 2022, 2022, CD013483.
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med.-Eur. J. Ultrasound 2017, 38, e16–e47.
- Yang, H.K.; Burns, P.N.; Jang, H.-J.; Kono, Y.; Khalili, K.; Wilson, S.R.; Kim, T.K. Contrast-enhanced ultrasound approach to the diagnosis of focal liver lesions: The importance of washout. Ultrasonography 2019, 38, 289–301.
- Minami, Y.; Kudo, M. Contrast-enhanced ultrasonography with Sonazoid in hepatocellular carcinoma diagnosis. Hepatoma Res. 2020, 2020.
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9.
This entry is offline, you can click here to edit this entry!
