Epigenetic dysregulation is an important feature for cancer initiation and progression. Long non-coding RNAs (lncRNAs) are transcripts that stably present as RNA forms with no translated protein and have lengths larger than 200 nucleotides. LncRNA can epigenetically regulate either oncogenes or tumor suppressor genes. Nowadays, the combined research of lncRNA plus protein analysis is gaining more attention. LncRNA controls gene expression directly by binding to transcription factors of target genes and indirectly by complexing with other proteins to bind to target proteins and cause protein degradation, reduced protein stability, or interference with the binding of other proteins. Various studies have indicated that lncRNA contributes to cancer development by modulating genes epigenetically and studies have been done to determine which proteins are combined with lncRNA and contribute to cancer development. In this review, we look in depth at the epigenetic regulatory function of lncRNAs that are capable of complexing with other proteins in cancer development.
- LncRNA
- protein
- epigenetic
- mechanism
- cancer
- development
- interation
1.Introduction
Among these non-coding transcripts, long non-coding RNAs (lncRNAs) have a length of 200 bp or more and are epigenetically involved with cancer development in various cells and tissues[1]. LncRNA can play a key role or act as a mediator in epigenetically controlled mechanisms of diverse cancer progression[2]. LncRNAs can regulate oncogenic or tumor suppressive gene expression by directly binding to the target gene or recruiting other transcriptional regulators to induce chromatin modification or DNA methylation in the nucleus[3][4] and by indirectly scaffolding or complexing with miRNA, mRNA, or proteins to control biological functions of the target protein in the cytoplasm[5][6].
2. Interaction
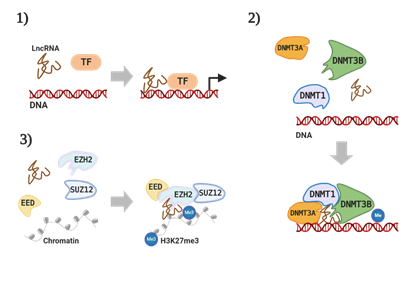
Recently, various studies and reviews have focused on the regulatory functions of lncRNA by searching for interactions with miRNAs that regulate target gene expression via binding to its Three prime untranslated region (3′UTR)[7]. However, many lncRNAs can regulate cancer-associated signaling pathways by directly binding to proteins that are related to cancer mechanisms[8]. It has been well-documented that lncRNAs interact with other proteins, including transcription factors (TFs)[9], DNA methyltransferases (DNMTs)[10], polycomb repressive complex 2 (PRC2)[11], RNA binding protein (RBP)[12], and heterogeneous nuclear ribonucleoprotein (hnRNP)[13], to regulate the target’s function in the mechanisms of cancer progression[14]. There have been certain major findings in the mechanisms by which lncRNA cooperates with other proteins: (1) lncRNA interacts with TFs to transcriptionally regulate target gene expression in cancer[9], (2) lncRNA binds to DNMTs such as, DNMT1, DNMT3A, and DNMT3B, causing the alteration of gene methylation[10], (3) lncRNA can remodel chromatin states by forming a complex with PRC2 that is comprised of embryonic ectoderm development (EED), suz12 polycomb repressive complex 2 subunit (Suz12), and methyltransferase enhancer of zeste homolog 2 (EZH2) (Figure 1)[11]. Following these findings, the complexes of various lncRNAs with many different types of proteins have been reported in various types of cancer as well as in different cancer-related processes [15].
Figure 1. Long non-coding RNA (lncRNA) interaction with RNA binding proteins (RBPs). (1) LncRNA regulates gene transcription by binding with transcription factors (TFs), (2) lncRNA induces DNA methyltransferases (DNMTs)-mediated methylation, and (3) lncRNA complex with polycomb repressive complex 2 (PRC2) to enhance H3K27me3. Figure is created with the program by BioRender.com.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12102925
References
- Julia Liz; Manel Esteller; lncRNAs and microRNAs with a role in cancer development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2016, 1859, 169-176, 10.1016/j.bbagrm.2015.06.015.
- Chunru Lin; Liuqing Yang; Long Noncoding RNA in Cancer: Wiring Signaling Circuitry. Trends in Cell Biology 2018, 28, 287-301, 10.1016/j.tcb.2017.11.008.
- Sun, M.; Nie, F.; Wang, Y.; Zhang, Z.; Hou, J.; He, D.; Xie, M.; Xu, L.; De, W.; Wang, Z.; et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016, 76, 6299–6310.
- Maruyama, R.; Suzuki, H. Long noncoding RNA involvement in cancer. BMB Rep. 2012, 45, 604–611.
- Tang, X.J.; Wang, W.; Hann, S.S. Interactions among lncRNAs, miRNAs and mRNA in colorectal cancer. Biochimie 2019, 163, 58–72.
- Klingenberg, M.; Gross, M.; Goyal, A.; Polycarpou-Schwarz, M.; Miersch, T.; Ernst, A.S.; Leupold, J.; Patil, N.; Warnken, U.; Allgayer, H.; et al. The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein L form a complex and coregulate genes linked to AKT signaling. Hepatology 2018, 68, 1817–1832.
- Volkmar Müller; Leticia Oliveira‐Ferrer; Bettina Steinbach; Klaus Pantel; Heidi Schwarzenbach; Interplay of lncRNA H19/miR‐675 and lncRNA NEAT1/miR‐204 in breast cancer. Molecular Oncology 2019, 13, 1137-1149, 10.1002/1878-0261.12472.
- Ammad Ahmad Farooqi; Rukset Attar; Muhammad Zahid Qureshi; Sundas Fayyaz; Muhammad Imran Sohail; Uteuliyev Yerzhan Sabitaliyevich; Sadykov Bolat Nurmurzayevich; Armida Yelekenova; Ilhan Yaylim; Nada Alaaeddine; et al. Interplay of long non-coding RNAs and TGF/SMAD signaling in different cancers. Cellular and Molecular Biology 2018, 64, 1-6, 10.14715/cmb/2017.64.15.1.
- Jinbao Wu; Xianmei Meng; Yanbin Jia; Jianyuan Chai; Jing Wang; Xiaohui Xue; Tong Dang; Long non-coding RNA HNF1A-AS1 upregulates OTX1 to enhance angiogenesis in colon cancer via the binding of transcription factor PBX3. Experimental Cell Research 2020, 393, 112025, 10.1016/j.yexcr.2020.112025.
- Jiali Wu; Zeyu Shuang; Jianfu Zhao; Hailin Tang; Peng Liu; Lijuan Zhang; XiaoMing Xie; Xiangsheng Xiao; Corrigendum to “Linc00152 promotes tumorigenesis by regulating DNMTs in triple-negative breast cancer” [Biomed Pharmacother. 97 (2018) 1275–1281]. Biomedicine & Pharmacotherapy 2020, 127, 110174, 10.1016/j.biopha.2020.110174.
- Junli Yan; Bibek Dutta; Yan Ting Hee; Wee-Joo Chng; Towards understanding of PRC2 binding to RNA. RNA Biology 2019, 16, 176-184, 10.1080/15476286.2019.1565283.
- Qiang Zhang; Yunzhen Wei; Zichuang Yan; Cheng Wu; Zhiqiang Chang; Yinling Zhu; Kun Li; Yan Xu; The characteristic landscape of lncRNAs classified by RBP–lncRNA interactions across 10 cancers. Molecular BioSystems 2017, 13, 1142-1151, 10.1039/c7mb00144d.
- Zheying Zhang; Chang Zhou; Yaya Chang; Zuoyang Zhang; Yuhan Hu; Fan Zhang; Yanxia Lu; Lin Zheng; Wenjuan Zhang; Xiaomin Li; et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Letters 2016, 376, 62-73, 10.1016/j.canlet.2016.03.022.
- Rong-Zhang He; Di-Xian Luo; Yin-Yuan Mo; Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes & Diseases 2019, 6, 6-15, 10.1016/j.gendis.2019.01.003.
- Maite Huarte; The emerging role of lncRNAs in cancer. Nature Medicine 2015, 21, 1253-1261, 10.1038/nm.3981.