Elaeodendron is a genus of tiny trees, shrubs, vines, and herbs consisting of about 23 species. It is used in traditional medicine and has a wide range of pharmacological activities. From the plants in this genus, flavonoids, terpenoids, cardiac glycosides, and cardenolides have been isolated. Preclinical investigations have also revealed antiviral, anti-HIV, anticancer, antiproliferative, antioxidant, antifungal, anti-inflammation, cytotoxic, anti-plasmodial, anti-arthritic, antibacterial, and anti-diabetic activities. Bioactive substances found in Elaedendron that function in a variety of ways are related to these biological processes.
- Elaeodendron
- Celastraceae
- cardenolides
1. Introduction
2. Traditional Uses
3. Bioactive Compounds from Elaeodendron Species
| Species | Isolated Compounds | Traditional Uses | Part Used | Reported Biological Activity | Reference |
|---|---|---|---|---|---|
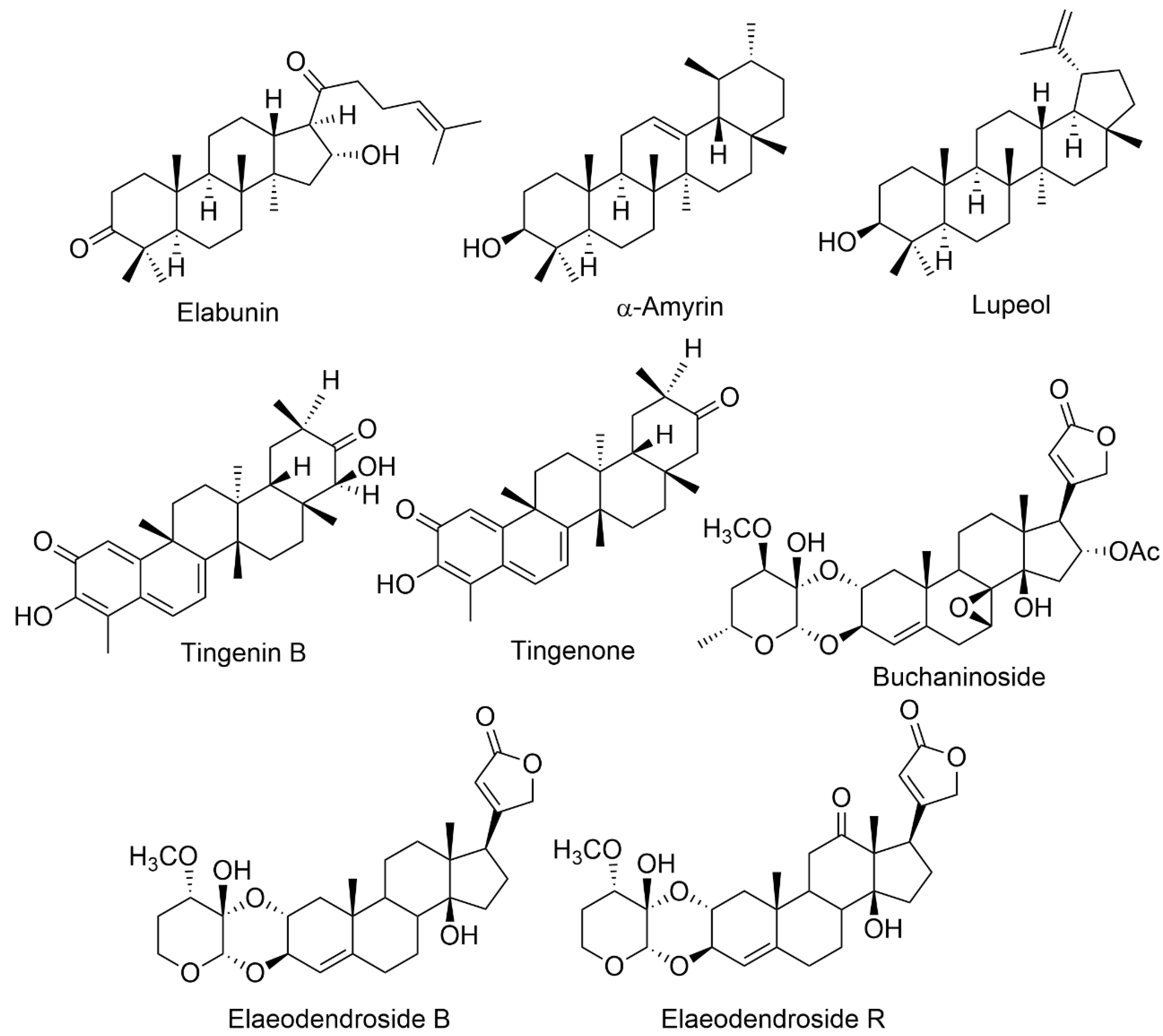
| E. buchananii | Elabunin; lupeol; 19α, 28-trihydroxyurs-12- en-23-oic acid; 3β, 11α, 3β-acetoxy-19α, 23, 28-trihydroxyurs-12-ene; 3-oxo-19α, buchaninoside; 19α-trihydroxyurs-12-en-23, 28-dioic acid; mutangin; methyl 3β-acetoxy-11α, 28 dihydroxyurs-12-en-24-oic acid | Fever, diarrhea gastrointestinal problems, bloody coughing, excessive uterine bleeding, infertility, syphilis, wounds, and leukemia | Leaves, Roots Bark | Anticancer, gastrointestinal disturbances, antimicrobial | [9][11] |
| E. croceum | 30-Hydroxylup 20(29)-en-3-one; (+)-6R,13R-11,11- dimethyl-1,3,8,10-tetrahydroxy-9-methoxy-peltogynan; galactitol; canophyllol; (−)-4′-0-methoxyepigallocatechin; tingenin B; ouratea-proanthocyanidin A; tingenone; 3-hydroxylupeol; 11α-hydroxy-β-amyrin; naringenin | Tuberculosis, blood in sputum, chest congestion, cough, sore throat, gastrointestinal system, fever | Stem bark | Anti-HIV, antibacterial, anti-arthritic, antimycobacterial, antifungal, antioxidant, anti-inflammatory, cytotoxic | [4][15] |
| E. glacum | 30-Hydroxylup-20(29)-en-3-one; tingenone; canophyllol; tingenin B; 3-hydroxylupeol; elaeodendroside; isocardenolide | Diabetes, sternutatories, nerve illnesses, swellings, headaches, emetic | Leaves, Root bark | Ani-diabetic, anti-snake-bite properties | [17] |
| E. orientale | Elaeodendroside F; elaeodendroside G; elaeodendroside T; elaeodendroside B; elaeodendroside C; elaeodendroside R; 20(22)-dienolid,6β,8β,11α,14β-tetrahydroxy-12-oxo-2α-O; 11α,14β-dihydroxy-2α-O;3β- O-(30α-methoxy-40-deoxy-50-dehydroxymethyl hexosulose)-card-4; 20(22)-dienolide, 3β-O-(20α,30β-methylendioxy)-40-desoxy-50-deshydroxymethyl-hexosu- lose]-card-4, 11α,14β-dihydroxy-2α-O; 3β-O-(30 α-methoxy-40-deoxy-50-dehydr-oxymethyl-hexosulose)-card-4; 20(22)-dienolide | chest infections, venereal illness, scorpion fish poisoning, astringent emetic, hypertension | Leaves, root bark | Anti-arthritic, antiproliferative, anticancer | [3][6] |
| E. schweinfurthianum | 3α-Hydroxyfriedelane; α-amyrin acetate; α-amyrin; 3-oxo-29-hydroxyfriedelane; β-sitosterol; lanosterol; stigmasterol; 3-oxofriedelane; 3-oxofriedelan-28-al | Fever | Roots | Antibacterial, anti-HIV, anti-plasmodial | [1] |
| E. schlechteranum | 4′,4″-Di-O-methyl-prodelphinidin; B4,3β,29-dihydroxyglutin-5-ene; 4′-O-methyl-epigallocatechin; tingenin B; 4′-O-methylgallocatechin; cangoronine methyl ester | Menstrual irregularities, anaemia, heart issues, high blood pressure, basic body discomfort, inflammatory disease, carbuncles boils, wounds | Roots, stem bark, root bark, leaf | Anti-HIV, anti-inflammatory | [23] |
| E. transvaalense | 4′-O-Methyl-epigallocatechin; canophyllal; (+)-, 11,11-dimethyl-1,3,8,10-trahydroxy-9-methoxypeltogynan; 6β-hydroxy-lup-20(30)-en-3-one; galactitol; hydroxylup-20(29)-ene-3-one; lup-20(29)-ene-30-hydroxy-3-one; Ψ-taraxastanonol; lup-20(30)-ene-3α,29-diol; lup-20(30)-ene-3α,29-diol; β-sitosterol; 3,28-dihydroxylbetuli-20(29)-ene; lup-20(30)-ene-3α,29-diollup-20(29)-ene-30-hydroxy-3-one; 4′-O-methyl-epigallocatechin; 3-oxo-28-hydroxylbetuli-20(29)-ene; 30-hydroxylup-20(29)- ene-3-one. | Diarrhea, stomachache, rashes, skin infections, inflammations, menorrhagia, women’s fertility issues, hypertension, HIV, sexually transmitted diseases (STDs). | Root bark | Anti-HIV, anti-inflammatory, antimicrobial, antioxidant, antimalarial, cytotoxic | [7][24][25] |
| E. xylocarpum | 3,25-Epoxy-olean-12-ene; 3β,21a-dihydroxyglut-5-ene; baruol; friedelin; cangoronine; cangoronine methyl ester; glutinol; 3β,29-dihydroxyglut-5-ene; wilforol E; 6β,30-dihydroxylup-20(29)-en-3-one; 6β-hydroxy-3-oxolup-20(29)-en-30-al; 3-oxolup-20(29)-en-30-oic acid; 3β,6β,20-trihydroxylupane; 11α,28-dihydroxylup-20(29)-en-3-one; 3- oxolup-20(29)-en-30-al; ochraceolide A; 12 3-oxo-30 hydroxylupane; 11 3-epiglochidiol; lupenone; botulin; 11 6β,20-dihydroxylupan-3-one; 16 lupan-3β-caffeate; 11 betulin-3β-caffeate; glochidiol; 3-epibetulin; betulone; 11α hydroxyglochidone; lupeol; rigidenol; nepeticin; glochidone; 25-hydroxylupeol; 15 3β,30-dihydroxylupane; 3-epinepeticin; 3b,29-Dihydroxy-olean-18-ene; 29-Hydroxy-3-oxo-olean-18-ene; 6b,29-Dihydroxy-3-oxo-olean-18-ene; 6b-Hydroxy-3-oxo-olean-18-ene; 3b,21a-Dihidroxy-olean-18-ene; 3b,6b-Dihidroxy-olean-18-ene; 21a-Hydroxy-3-oxo-olean-18-ene; 3b,11a,28-Trihydroxy-olean-18-ene; 29-Acetoxy-3-oxo-olean-18-ene; 3b,21a-Diacetoxy-olean-18-ene; 3b-Acetoxy-6b-hydroxy-olean-18-ene; 6β,30-Dihydroxylup-20(29)-en-3-one; 6β-Hydroxy-3-oxolup-20(29)-en-30-al; 3-Oxolup-20(29)-en-30-oic acid; 3β,6β,20-Trihydroxylupane; 1β,3α,28-Trihydroxylup-20(29)-ene; 11α,28-Dihydroxy-3-oxolup-20(29)-ene; 3β,28-Di-O-octanoylbetulin; 28-O-(1-Naphthoyl)botulin; 3β,28-Di-O-(1-naphthoyl)botulin; 28-Oacetyl-3β,20,29-trihydroxylupane; 28-O-acetyl20R,29-epoxy-3β-hydroxylupane; 2 (28-O-acetyl-3β-hydroxylup-20(29)-en30-al; 3β,30-di-O-acetyllup-20(29)-ene; 2-bromo-3-oxolup-20(29)-ene;11α-O-acetyl-3-oxolup-20(29)-ene;11α-O-Acetyl-30-chloro-3-oxolup-1,20(29)-diene | Stimulant | Root bark | Anti-HIV | [26][27] |
4. Pharmacological Properties of Elaeodendron Species
4.1. Antioxidant Activity
4.2. Anti-inflammatory Activity
4.3. Antibacterial Activity
Khumalo et al. examined the antimicrobial property of E. transvaalense stem bark extracts and components in methanol and dichloromethane. 6β-hydroxy-lup-20(29)-ene-3-one,4′-O-methylepigallocatechin, lup-20(30)-ene-3α,29-diol, and 30-hydroxylup-20(29)-ene-3-one were tested against Salmonella typhimurium, Staphylococcus epidermidis [31] Staphylococcus aureus, Escherichia coli, Shigella sonnei, and Pseudomonas aeruginosa using a micro-titer plate broth two-fold serial dilution experiment with ciprofloxacin as the control sample. The extract and compounds had moderate antibacterial activity, with minimum inhibitory concentration values 0.1 mg/mL to 1.7 mg/mL. Using the serial broth microdilution assay and ciprofloxacin as a positive control. Mamba et al. investigated the antimicrobial activities of E. transvaalense bark ethanol extracts and the molecules 4′-O-methyl-epigallocatechin, lup-20(30)-ene-3,29-diol and lup-20(29)-ene-30-hydroxy-3-one isolated from the plant against Neisseria gonorrhoeae, Oligella ureolytica, and Gardnerella vaginalis. MIC values for the extracts and compounds varied from 1.6 mg/mL to 12.5 mg/mL, whereas the positive control had a MIC of 0.01 mg/mL [16]. McGaw et al. used disc-diffusion and micro-dilution assays to test the antimicrobial activity of E. transvaalense bark aqueous and hexane ethanol extracts against Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, and Escherichia coli with neomycin as a positive control. Water and ethanolic extracts were potent against Bacillus subtilis and Staphylococcus aureus, with MICs ranging from 0.1 mg/mL to 0.8 mg/mL [32]. Using the agar dilution method, Tshikalanga et al. investigated the antimicrobial activities of E. transvaalense chloroform and aqueous bark extracts against Enterobacter cloacae, Enterobacter aerogenes, Bacillus pumilus, Bacillus cereus, Klebsiella pneumoniae, Bacillus subtilis, and Escherichia coli. The extracts had MIC values between 20 mg/mL to 50 mg/mL against Bacillus cereus, Bacillus pumilus, Bacillus subtilis, and Staphylococcus aureus [7].
4.4. Cytotoxic Activity and Antiproliferative Activity
4.5. Anti-fungal Activity
4.6. Anti-HIV Activity
4.7. Anti-plasmodial Activity
4.8. Larvicidal Activities
4.9. Anti-pyretic Activities
4.10. Hypoglycaemic Activity
4.11. Anti-arthritic Activity
4.12. Anti-diabetic Activity
This entry is adapted from the peer-reviewed paper 10.3390/app122412618
References
- Opiyo, S.A.; Manguro, L.O.A.; Owuor, P.O.; Ateka, E.M. Triterpenes from Elaeodendron schweinfurthianum and their antimicrobial activities against crop pathogens. Am. J. Chem. 2017, 7, 97–104.
- Archer, R.H.; Van Wyk, A.E. A taxonomic revision of Elaeodendron Jacq. (Cassinoideae: Celastraceae) in Africa. S. Afr. J. Bot. 1998, 64, 93–109.
- Cao, S.; Brodie, P.J.; Miller, J.S.; Ratovoson, F.; Callmander, M.W.; Randrianasolo, S.; Rakotobe, E.; Rasamison, V.E.; Suh, E.M.; TenDyke, K.; et al. Antiproliferative Cardenolides of an Elaeodendron Sp. from the Madagascar rain forest. J. Nat. Prod. 2007, 70, 1064–1067.
- Yelani, T.; Hussein, A.A.; Meyer, J.J.M. Isolation and identification of poisonous triterpenoids from Elaeodendron croceum. Nat. Prod. Res. 2010, 24, 1418–1425.
- Kubo, I.; Fukuhara, K. Elabunin, a new cytotoxic triterpene from an east African medicinal plant, Elaeodendron buchananii. J. Nat. Prod. 1990, 53, 968–971.
- Osorio, A.A.; López, M.R.; Jiménez, I.A.; Moujir, L.M.; Rodríguez, M.L.; Bazzocchi, I.L. Elaeodendron orientale as a source of cytotoxic Cardenolides. Phytochemistry 2014, 105, 60–67.
- Tshikalange, T.E.; Hussein, A.A.; Meyer, J.J.M. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J. Ethnopharmacol. 2005, 96, 515–519.
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu Sub-County, Nyamira County, Kenya. PLoS ONE 2017, 12, e0185722.
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Azeze Tebo, T.; Nteziyaremye, P.; Nyambura Karanja, L.; Jepchirchir, A.; et al. Medicinal plants used in traditional management of cancer in Uganda: A review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid.-Based Complement Altern. Med. 2020.
- Lemmens, R.H.M.J.; Louppe, D.; Oteng-Amoako, A.A. Timbers 2. Plant Resources of Tropical Africa-PROTA; PROTA Foundation: Wageningen, The Netherlands, 2012; Volume 2, ISBN 9789290814955.
- Odak, J.A.; Manguro, L.O.A.; Wong, K.C. New compounds with antimicrobial activities from Elaeodendron buchananii stem bark. J. Asian Nat. Prod. Res. 2018, 20, 510–524.
- Lemmens, R.H.M.J. Elaeodendron buchananii (PROTA)-PlantUse English. Available online: https://uses.plantnet-project.org/e/index.php?title=Elaeodendron_buchananii_(PROTA)&mobileaction=toggle_view_desktop (accessed on 21 September 2022).
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kiama, S.G. Antimalarial herbal remedies of Msambweni, Kenya. J. Ethnopharmacol. 2010, 128, 424–432.
- Tsujino, Y.; Ogoche, J.I.J.; Tazaki, H.; Fujimori, T.; Mori, K. Buchaninoside, a steroidal glycoside from Elaeodendron buchananii. Phytochemistry 1995, 40, 753–756.
- Maroyi, A. Medicinal uses, phytochemistry, pharmacology and toxicological properties of Elaeodendron croceum. Trop. J. Pharm. Res. 2019, 18, 669–676.
- Mamba, P.; Adebayo, S.A.; Tshikalange, T.E. Anti-microbial, anti-inflammatory and HIV-1 reverse transcriptase activity of selected South African plants used to treat sexually transmitted diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1870–1876.
- Lanjhiyana, S.; Garabadu, D.; Ahirwar, D.; Bigoniya, P.; Chand, A. Antidiabetic activity of methanolic extract of stem bark of Elaeodendron glaucum Pers. in alloxanized rat model. Adv. Appl. Sci. Res. 2011, 2, 47–62.
- Tsanuo, M.K.; Hassanali, A.; Jondiko, I.J.O.; Torto, B. Mutangin, a dihydroagarofuranoid sesquiterpene insect antifeedant from Elaeodendron buchananii. Phytochemistry 1993, 34, 665–667.
- Jayaweera, D.M.A. Medicinal Plants (Indigenous and Exotic) Used in Ceylon: Part II; The National Science Foundation: Colombo, Sri Lanka, 2006.
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354.
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34.
- Omosa, L.K.; Amugune, B.; Mutai, P.; Karumu, E.; Mukungu, N.; Induli, M.; Kama-Kama, F.; Kuete, V. Rapid screening using GIBEX screens-to-nature system of ethnomedicinal plants from Ngong Forest, Kenya for potency against infectious diseases and antioxidant activities: A qualitative study. Pharmacogn. Commun. 2019, 9, 59–74.
- Maregesi, S.M.; Hermans, N.; Dhooghe, L.; Cimanga, K.; Ferreira, D.; Pannecouque, C.; Berghe, D.A.V.; Cos, P.; Maes, L.; Vlietinck, A.J.; et al. Phytochemical and biological investigations of Elaeodendron schlechteranum. J. Ethnopharmacol. 2010, 129, 319–326.
- Maroyi, A.; Semenya, S.S. Medicinal uses, phytochemistry and pharmacological properties of Elaeodendron transvaalense. Nutrients 2019, 11, 545.
- Núñez, M.J.; Ardiles, A.E.; Martínez, M.L.; Torres-Romero, D.; Jiménez, I.A.; Bazzocchi, I.L. Triterpenoids from Cassine xylocarpa and Celastrus vulcanicola (Celastraceae). Phytochem. Lett. 2013, 6, 148–151.
- Osorio, A.A.; Muñóz, A.; Torres-Romero, D.; Bedoya, L.M.; Perestelo, N.R.; Jiménez, I.A.; Alcamí, J.; Bazzocchi, I.L. Olean-18-ene triterpenoids from Celastraceae species inhibit HIV replication targeting NF-KB and Sp1 dependent transcription. Eur. J. Med. Chem. 2012, 52, 295–303.
- Araujo-León, J.A.; Cantillo-Ciau, Z.; Ruiz-Ciau, D.V.; Coral-Martínez, T.I. HPLC profile and simultaneous quantitative analysis of tingenone and pristimerin in four Celastraceae species using HPLC-UV-DAD-MS. Rev. Bras. Farmacogn. 2019, 29, 171–176.
- Odeyemi, S.W.; Afolayan, A.J. Biological activities and phytochemical screening of Elaeodendron croceum (Thunb.) DC. leaves and stem barks extracts. Int. J. Phytomedicine 2017, 9, 566.
- Olaokun, O.O.; Mkolo, N.M.; King, P.H. Inhibition of 5-lipoxygenase and phytochemical content of five South African medicinal plants. S. Afr. J. Bot. 2018, 115, 326–327.
- Elisha, I.L.; Dzoyem, J.P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement Altern. Med. 2016, 16, 307.
- Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.E. Antimicrobial lupenol triterpenes and a polyphenol from Elaeodendron transvaalense, a popular Southern African medicinal bark. S. Afr. J. Bot. 2019, 122, 518–521.
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263.
- Prinsloo, G.; Meyer, J.J.M.; Hussein, A.A.; Munoz, E.; Sanchez, R. A Cardiac glucoside with in vitro anti-HIV activity isolated from Elaeodendron croceum. Nat. Prod. Res. 2010, 24, 1743–1746.
- Elisha, I.L.; Dzoyem, J.P.; Botha, F.S.; Eloff, J.N. The efficacy and safety of nine South African medicinal plants in controlling Bacillus anthracis sterne vaccine strain. BMC Complement Altern. Med. 2016, 16, 5.
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 133.
- Prinsloo, G.; Meyer, J.J.M.; Hussein, A.A. Anti-HIV activity of a cardiac glycoside isolated from Elaeodendron croceum. S. Afr. J. Bot. 2007, 73, 308.
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1988, 20, 309–321.
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434.
- Bessong, P.O.; Obi, C.L.; Andréola, M.L.; Rojas, L.B.; Pouységu, L.; Igumbor, E.; Meyer, J.J.M.; Quideau, S.; Litvak, S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J. Ethnopharmacol. 2005, 99, 83–91.
- Nethengwe, M.F.; Opoku, A.R.; Dludla, P.V.; Madida, K.T.; Shonhai, A.; Smith, P.; Singh, M. Larvicidal, antipyretic and antiplasmodial activity of some Zulu medicinal plants. J. Med. Plants Res. 2012, 6, 1255–1262.
- Deutschländer, M.S.; van de Venter, M.; Roux, S.; Louw, J.; Lall, N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J. Ethnopharmacol. 2009, 124, 619–624.
