Root exudates, as an important form of material input from plants to the soil, regulate the carbon input and efflux of plant rhizosphere soil and play an important role in maintaining the carbon and nutrient balance of the whole ecosystem. Root exudates are notoriously difficult to collect due to their underlying characteristics (e.g., low concentration and fast turnover rate) and the associated methodological challenges of accurately measuring root exudates in native soils. As a result, up until now, it has been difficult to accurately quantify the soil organic carbon input from root exudates to the soil in most studies. The contribution and ecological effects of root exudates to soil organic carbon input and efflux have been paid more and more attention.
- rhizosphere
- root exudates
- soil organic carbon
- soil microbes
1. Introduction
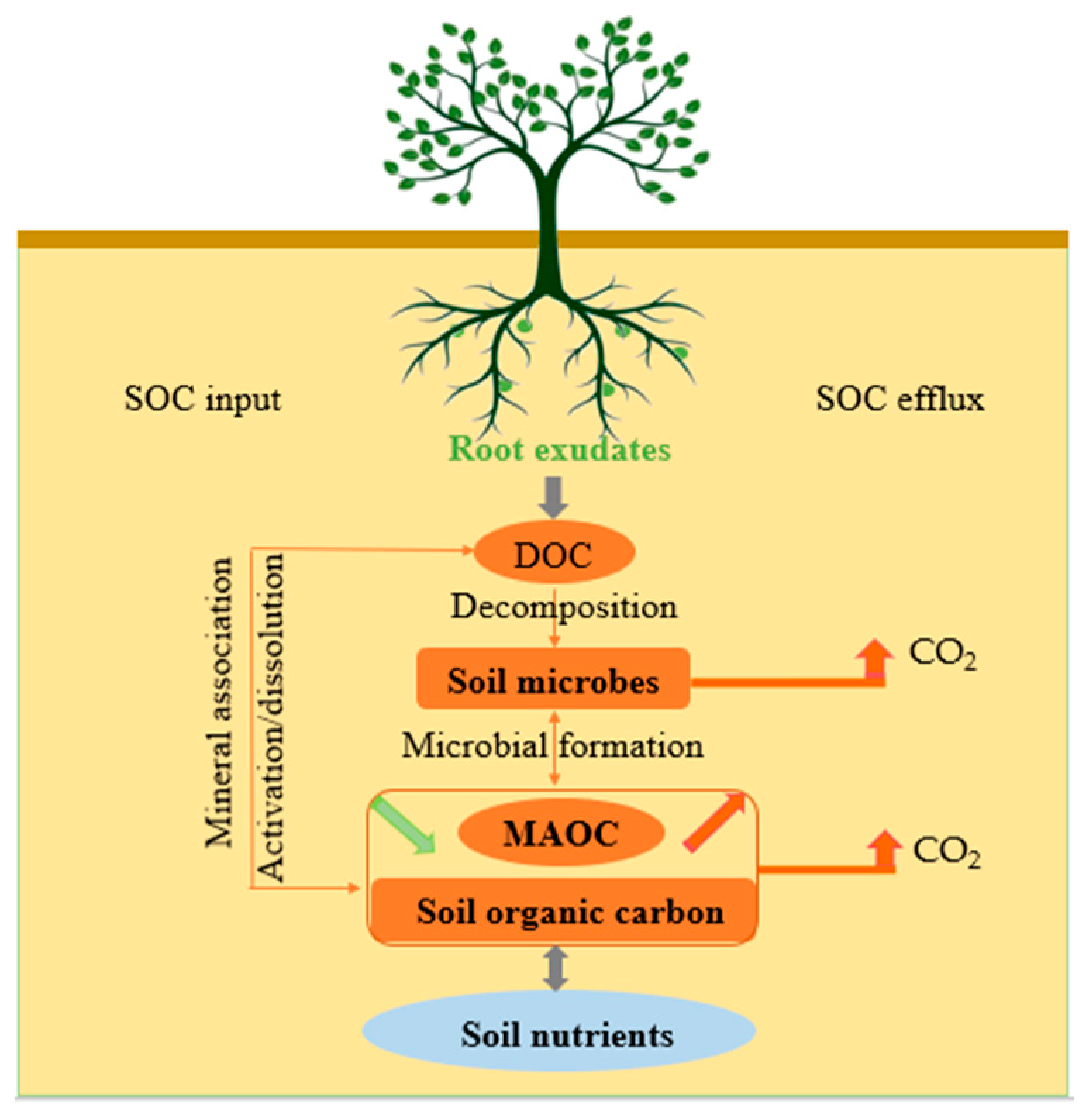
2. Root Exudates and Soil Organic Carbon Input
3. Root Exudates and Soil Organic Carbon Efflux
This entry is adapted from the peer-reviewed paper 10.3390/plants12030630
References
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68.
- Abdullahi, A.C.; Siwar, C.; Shaharudin, M.I.; Anizan, I. Carbon Sequestration in Soils: The Opportunities and Challenges. Carbon Capture Util. Sequestration 2018.
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273.
- Yu, W.; Huang, W.; Weintraub-Leff, S.R.; Hall, S.J. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Bio. Biochem. 2022, 172, 108756.
- Chen, L.; Fang, K.; Wei, B.; Qin, S.; Feng, X.; Hu, T.; Ji, C.; Yang, Y.; Cleland, E. Soil carbon persistence governed by plant input and mineral protection at regional and global scales. Ecol. Lett. 2021, 24, 1018–1028.
- Sokol, W.N.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246.
- Hicks, P.; Caitlin, E.; Bird, J.; Castanha, C.; Hatton, P.J.; Torn, M.S. Long term decomposition: The influence of litter type and soil horizon on retention of plant carbon and nitrogen in soils. Biogeochemistry 2017, 134, 5–16.
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Ann. Rev. Ecol. 2017, 48, 419–445.
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618.
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12.
- Huang, J.; Liu, W.; Yang, S.; Yang, L.; Peng, Z.; Deng, M.; Xu, S.; Zhang, B.; Ahirwal, J.; Liu, L. Plant carbon inputs through shoot, root, and mycorrhizal pathways affect soil organic carbon turnover differently. Soil Biol. Biochem. 2021, 160, 108322.
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65.
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718.
- Freschet, G.T.; Roumet, C.; Treseder, K. Sampling roots to capture plant and soil functions. Funct. Ecol. 2017, 31, 1506–1518.
- Huang, J.; Liu, W.; Deng, M.; Wang, X.; Wang, Z.; Yang, L.; Liu, L. Allocation and turnover of rhizodeposited carbon in different soil microbial groups. Soil Biol. Biochem. 2020, 150, 107973.
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Penuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696.
- Xiao, C.; Yang, F.; Zhou, Y.; Su, J.; Liang, Y.; Pei, Z. Research progress on belowground carbon input and efflux processes in terrestrial ecosystems. Chin. Bull. Bot. 2017, 52, 652–668.
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biol. Biochem. 2020, 146, 107817.
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595.
- Zhang, Y.; Zhu, L.; Cheng, Y.; Xing, Q.; Lan, Y. Current situation and trend of root exudates research-knowledge mapping analysis based on citespace. Jiangsu Agric. Sci. 2022, 50, 34–45.
- Yin, H.; Zhang, Z.; Liu, Q. Root exudates and their ecological consequences in forest ecosystems: Problems and perspective. Chin. J. Plant Ecol. 2018, 42, 1055–1070.
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Bio. Biochem. 2014, 77, 69–80.
- Li, J.; Fan, M.; Shang-Guan, Z. Research progress on main ecological functions of plant root exudates. Chin. Bull. Bot. 2020, 55, 788–796.
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157.
- Li, Y.; Yang, F.; Han, P.; Zhou, W.; Wang, J.; Yan, X.; Lin, J. Research progress on the mechanism of root exudates in response to abiotic stresses. Chin. J. Appl. Environ. Biol. 2021, 28, 1384–1392.
- Zhou, S.; Lin, J.; Wang, P.; Zhu, P.; Zhu, B. Resistant soil organic carbon is more vulnerable to priming by root exudate fractions than relatively active soil organic carbon. Plant Soil 2022, in press.
- Pett-Ridge, J.; Firestone, M.K. Using stable isotopes to explore root-microbe-mineral interactions in soil. Rhizosphere 2017, 3, 244–253.
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.; Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630.
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front. Environ. Sci. 2018, 6, 140.
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779.
- Gao, X. Root Exudates of Dominant Plants and the Effects of Their Main Components on Soil Microorganisms in Stipa Breviflora Desert Steppe. Doctoral Dissertation, Inner Mongolia Agricultural University, Huhhot, China, 2017.
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2021, 110, 21–33.
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Bio. Biochem. 2018, 120, 246–259.
- Guyonnet, J.P.; Cantarel, A.A.M.; Simon, L.; Haichar, F.E. Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol. Evol. 2018, 8, 8573–8581.
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271.
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310.
- Wang, R.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J. Ecol. 2021, 109, 3699–3709.
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905.
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371.
- Zhou, J.; Guillaume, T.; Wen, Y.; Blagodatskaya, E.; Shahbaz, M.; Zeng, Z.; Peixoto, L.; Zang, H.; Kuzyakov, Y. Frequent carbon input primes decomposition of decadal soil organic matter. Soil Biol. Biochem. 2022, 175, 108850.
- Henneron, L.; Cros, C.; Picon-Cochard, C.; Rahimian, V.; Fontaine, S. Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J. Ecol. 2019, 108, 528–545.
- Li, J.; Zhang, R.; Cheng, B.; Ye, L.; Li, W.; Shi, X. Effects of nitrogen and phosphorus additions on decomposition and accumulation of soil organic carbon in alpine meadows on the Tibetan Plateau. Land Degrad. Dev. 2021, 32, 1467–1477.
- Han, M.; Sun, L.; Gan, D.; Fu, L.; Zhu, B. Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol. Biochem. 2020, 151, 108019.
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112.
- Zhang, Q.; Feng, J.; Li, J.; Huang, C.; Shen, Y.; Cheng, W.; Zhu, B. A distinct sensitivity to the priming effect between labile and stable soil organic carbon. New Phytol. 2022, 237, 88–99.
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 2015, 21, 2082–2094.
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480.
- Qiao, N.; Schaefer, D.; Blagodatskaya, E.; Zou, X.; Xu, X.; Kuzyakov, Y. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Chang. Biol. 2014, 20, 1943–1954.
- Wang, R.; Bicharanloo, B.; Shirvan, M.B.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. A novel 13C pulse-labelling method to quantify the contribution of rhizodeposits to soil respiration in a grassland exposed to drought and nitrogen addition. New Phytol. 2021, 230, 857–866.
