Currently, algae arouse a growing interest in the pharmaceutical and cosmetic area due to the fact that they have a great diversity of bioactive compounds with the potential for pharmacological and nutraceutical applications. Due to lifestyle modifications brought on by rapid urbanization, diabetes mellitus, a metabolic illness, is the third largest cause of death globally. The hunt for an efficient natural-based antidiabetic therapy is crucial to battling diabetes and the associated consequences due to the unfavorable side effects of currently available antidiabetic medications. Finding the possible advantages of algae for the control of diabetes is crucial for the creation of natural drugs.
Many of algae’s metabolic processes produce bioactive secondary metabolites, which give algae their diverse chemical and biological features. Numerous studies have demonstrated the antioxidant and antidiabetic benefits of algae, mostly by blocking carbohydrate hydrolyzing enzyme activity, such as -amylase and -glucosidase. Additionally, bioactive components from algae can lessen diabetic symptoms in vivo. Therefore, the current review concentrates on the role of various secondary bioactive substances found naturally in algae and their potential as antioxidants and antidiabetic materials, as well as the urgent need to apply these substances in the pharmaceutical industry
1. Introduction
The prevalence of diabetes has increased rapidly over the past few years, mainly in low- to middle-income countries, and has become one of the major causes of premature death worldwide. According to International Diabetes Federation (IDF) [
1], there are about 537 million individuals living with diabetes, and about 316 million persons suffer from weakened glucose tolerance and augmented risk of diabetes. These statistics are predicted to increase to 643 million by 2030, and in less than a quarter of a century, it is predicted that there will be 783 million people suffering from this disease in the absence of rapid and accurate prevention procedures [
1].
Diabetes mellitus is an extended metabolic disorder of several etiologies, characterized by chronic hyperglycemia with the ailment of carbohydrate, fat, and also protein metabolism, which typically results from an absolute or relative lack of insulin, impaired effectiveness of insulin action, or tissue insensitivity to insulin [
2].
Insulin is a hormone produced by the pancreatic β-cells that functions to maintain the strict control of blood glucose. This hormone enables the tissues and cells of the body to utilize glucose for energy. If insulin is absent or its action is impaired due to tissue insensitivity, cells, and tissues are unable to uptake glucose, which results in its accumulation in the blood, and consequently, diabetes symptoms occur [
3].
Symptoms of diabetes often go unobserved because they can be attributed to many other causes, and some patients fail to notice cautioning signs or practice definite indicative symptoms [
4]. However, possible symptoms of diabetes may include the following: unexplained weight loss, excessive thirst (polydipsia), excessive urination (polyuria) and dehydration, excessive hunger, general fatigue, blurred vision, nearsightedness or other vision problem, and vaginal infection. The control of hyperglycemia (abnormally high glucose) is very important in the treatment of all forms of diabetes because, in the long term, acute and chronic complications can happen when the blood glucose concentration is not normalized [
2].
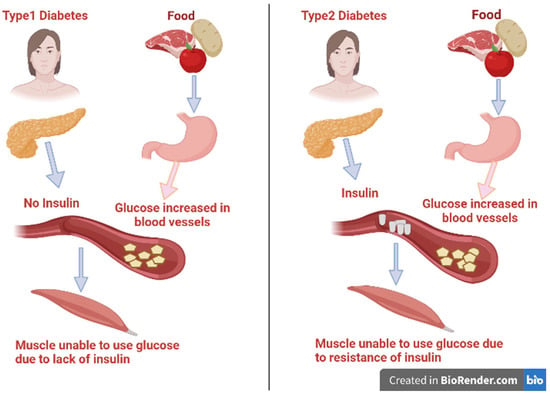
There are various categories of diabetes: type 1 diabetes, type 2 diabetes, gestational diabetes mellitus
, and other specific categories of diabetes that have several causes, such as pancreatic or drug-related diseases, monogenic diabetic syndrome, and chemical inducers [
5]. The two main types of diabetes mellitus are illustrated in
Figure 1. Type 1 diabetes is also identified as insulin-dependent diabetes mellitus (IDDM). It represents about 10% of all cases of diabetes and was previously known as juvenile-onset diabetes as it usually occurs in persons under 40 years of age [
6,
7]. In this type, there is usually a lack of the secretion of insulin as a result of disorders affecting and deteriorating the pancreatic β-cells. It is conveyed to have a genetic inheritance tendency and an autoimmune basis that lead to β-cell destruction due to the presence of anti-insulin antibodies [
5]. As a result of insulin insufficiency, the body will be forced to burn fats for energy instead of glucose, resulting in a toxic byproduct called ketones under severe hyperglycemia [
8]. Patients of IDDM need a daily dosage of insulin to live and avoid the progress of ketoacidosis.
Figure 1. Types of diabetes mellitus and their syndrome.
Alternatively, type 2 affects about 95% of people diagnosed with diabetes mellitus and are 40 years and above in age [
9]. It happens as a result of the progressive loss of β-cells secreting insulin or tissue insensitivity to absorb insulin that impairs insulin action [
10]. Fast food globalization, unhealthy eating patterns, and inactivity could lead to an increase in body mass, and these may be the main causes of diabetes.
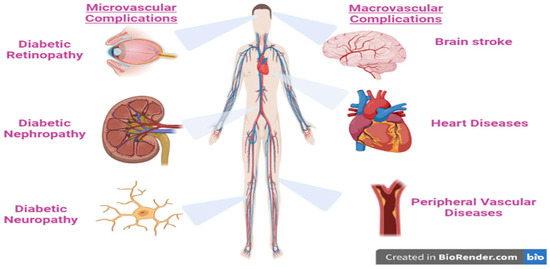
2. Factors That Contribute to Diabetes and Its Complications
The majority of diabetes types across the world may be correlated to modern diets, sedentary lifestyles, and obesity. The mortality associated with diabetes is mainly a result of the augmented danger of several complications of this disease. Even though diabetes is primarily defined by chronic hyperglycemia, many diabetic patients, particularly those with type 2, have elevated blood pressure (hypertension), chronic high levels of insulin (hyperinsulinemia), and abnormal levels of cholesterol, triglycerides, and/or other blood lipids (hyperlipidemia). In addition, lipoprotein abnormalities are some of the most prevalent problems associated with type 2 diabetes [
11]. These complications are strictly related to the disease disorders, as well as to the procedures used to diagnose and treat them (
Figure 2).
Figure 2. The major complication of type 2 diabetes mellitus.
3. Oxidative Stress Linked to Diabetes
Biochemical processes in the body may produce intermediate products called reactive oxygen species (ROS). Among ROS, harmful free radicals have one or more unpaired electrons that make them very reactive with other molecules. Excess ROS may lead to an imbalance in its metabolism and in the body’s ability to detoxify or counteract the harmful oxidant effects of the free radicals [
12,
13]. This condition is known as oxidative stress, which depends on the balance between ROS production and antioxidant defenses [
14]. Oxidative stress is thus the result of the imbalance between the formation and neutralization of reactive oxygen and nitrogen species [
15,
16]. Electron transfers to O
2 are catalyzed by oxidase enzymes to produce chemical energy or oxidation of substrates. These enzymes are potential sources of reduced Cu
2+ derivatives in biological settings; they also produce O
2•− during catalysis [
17,
18,
19]. The mitochondrial electron transport chain reduces O
2 to O
2•− [
20,
21]. Dismutase enzymes reduce O
2•− radicals to form hydrogen peroxide (H
2O
2) and/or further react to form the hydroxyl secondary radical (
•−OH) as another type of the ROS [
17,
19]. Although the cause-effect relationship remains unsure, there appears to be a strong correlation between mitochondrial dysfunction and chronic metabolic diseases, such as obesity [
17] and diabetes mellitus (
Figure 3) [
22]. This relationship results in oxidative damage to cellular components in the form of lipid peroxidation, protein denaturation or DNA conjugation, and finally, cell death [
23]. As a result of the aforementioned reasons, oxidative stress has been related to many diseases, such as cancer, post-ischemic and neural degradation, Parkinson’s and Alzheimer’s diseases, acquired immune deficiency syndrome (AIDS), aging, and cardiovascular diseases [
24].
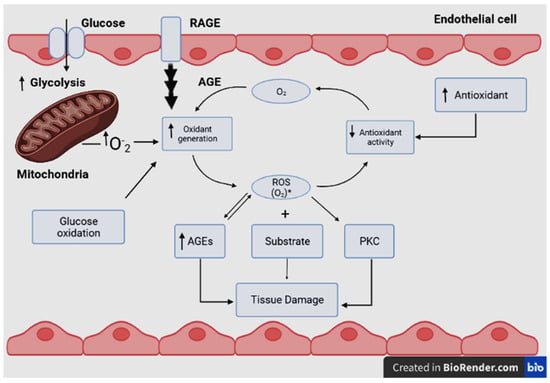
Figure 3. The relationship between rates of oxidant generation, antioxidant activity, oxidative stress, and oxidative damage in diabetes. O2•− represents various forms of ROS. The overall rate of formation of oxidative products, which lead to oxidative tissue damage, is dependent on ambient levels of both O2•− and substrate. Increased generation of O2•− depends on several sources, including glucose autoxidation, increased mitochondrial superoxide production, and increased endoplasmic reticulum superoxide production, as well as the result of the receptor for advanced glycosylation end product activation. O2•− deactivation is reduced because antioxidant defenses are compromised in diabetes. Note that oxidative stress also promotes other hyperglycemia-induced mechanisms of tissue damage. Additionally, oxidative stress activates protein kinase C (PKC) and accelerates the formation of advanced glycosylation end products (AGEs), RAGE: Receptor for AGEs.
This entry is adapted from the peer-reviewed paper 10.3390/life13020460