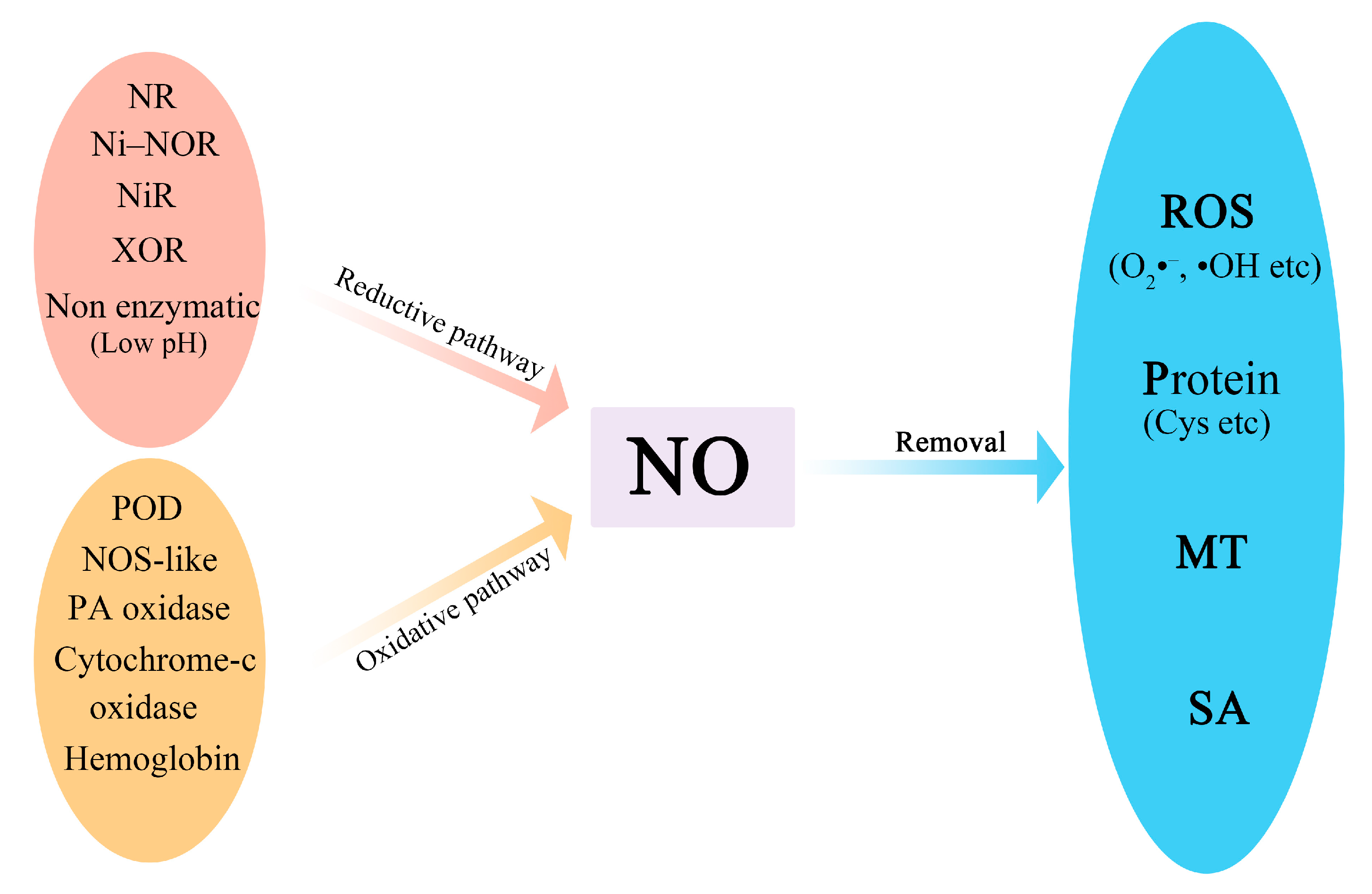
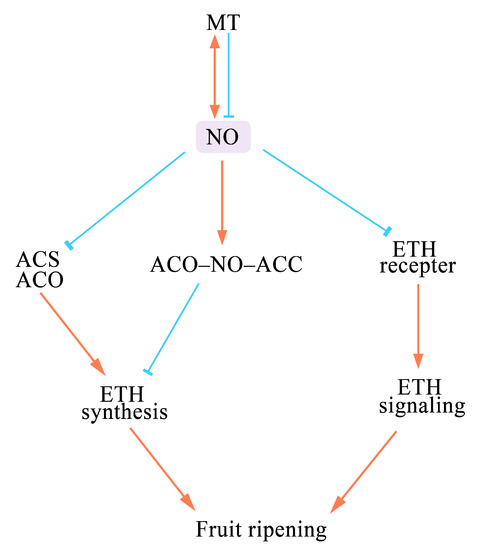
Nitric oxide (NO) is a gaseous free radical that has been become a potential tool to maintain the quality of postharvest horticultural produce (such as fruits). It plays important roles in delaying ripening, alleviating chilling injury, preventing browning, and enhancing disease resistance. The regulatory function of NO is achieved through the post-transcriptional modification of proteins, such as tyrosine nitration, S-nitrosylation, and nitroalkylation. Secondly, NO can also induce the expression of stress-related genes by synergistically interacting with other signaling substances, such as Ca2+, ethylene (ETH), salicylic acid (SA), and jasmonic acid (JA).
- nitric oxide
- fruits and vegetables
- ripening
1. Introduction
2. Effects of NO on Fruit Ripening
| Fruits | Best Treatment | Effects | References |
|---|---|---|---|
| Blueberry (Blue Cuinex, Blue Chip and Misty) |
Blue Cuinex: 1 μL L−1 1-MCP + 1 mM GSNO Misty: 1 μL L−1 1-MCP Blue Chip: Not affected by treatment. |
Maintained higher firmness, malic acid, citric acid, ascorbic acid, and glutathione contents for 14 d at 4 °C. | [17] |
| Tomato (Elpida) | 1 mM GSNO + 0.5 μL L−1 1-MCP | Delayed fruit softening, reduced the ETH synthesis significantly. | [18] |
| Red raspberry (Rubus idaeus L.) |
15 μM NO solution for 2 min (immersed in) | Reduced ETH production, respiratory intensity, ROS contents and increased the contents of flavonoids, anthocyanin, rutin, influenced metabolism of sugars. | [19] |
| Sweet pepper | 160 μM (5 ppm) NO gas for 1 h | Delayed the ripening of fruit, decreased lipid peroxidation, and increased antioxidant capacity, ascorbate content. | [20][21][22] |
| Banana (Brazil) | 5 mM SNP solution | Reduced ETH production, inhibited degreening of the peel, and delayed softening of the pulp. Inhibited the activity of ACO. | [23] |
| Papaya (Sui you 2) | 60 mL L−1 NO fumigated for 3 h | Suppressed ETH formation and respiratory rate (CO2 levels), reduced weight loss, maintained firmness, and delayed changes in peel color and soluble solid contents during 20 d of storage. | [24] |
| Wax apple (Syzygium samarangense) | 10 μL L−1 NO fumigated for 2 h | Lower rate of weight loss, a softening index, and loss of firmness during storage. Decreased total lignin content. | [25] |
| Tomato (Lichun) | 0.1 mM L-NAME solutions for 0.5 min | Decreased endogenous ETH release and delayed the breaker stage of fruits. | [26] |
| Peach (Dahong) | 15 μL L−1 NO + 20 μL L−1 H2S fumigated for 20 min | Inhibited ripening of peach fruits, reduced softening related enzymes activities, ETH production, ACC content, ACC synthase, and oxidase activities. | [27] |
| Water bamboo shoots (Zizania latifolia) | 30 μL L−1 NO fumigated for 4 h | Suppressed the softening and lignification effectively. | [28] |
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9020135
References
- Chen, T.; Qin, G.Z.; Tian, S.P. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226.
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.Y.; Evanich, D.J.; Shi, Y.N.; Xu, Y.M.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364.
- Foresi, N.; Mayta, M.L.; Lodeyro, A.F.; Scuffi, D.; Correa-Aragunde, N.; Garcia-Mata, C.; Casalongue, C.; Carrillo, N.; Lamattina, L. Expression of the tetrahydrofolate-dependent nitric oxide synthase from the green alga Ostreococcus tauri increases tolerance to abiotic stresses and influences stomatal development in Arabidopsis. Plant J. 2015, 82, 806–821.
- Dean, J.V.; Harper, J.E. The Conversion of Nitrite to Nitrogen Oxide(s) by the Constitutive NAD(P)H-Nitrate Reductase Enzyme from Soybean. Plant Physiol. 1988, 88, 389–395.
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. Febs Lett. 2000, 468, 89–92.
- Salgado, I.; Martinez, M.C.; Oliveira, H.C.; Frungillo, L. Nitric oxide signaling and homeostasis in plants: A focus on nitrate reductase and S-nitrosoglutathione reductase in stress-related responses. Braz. J. Bot. 2013, 36, 89–98.
- He, H.Y.; He, L.F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant. 2020, 170, 218–226.
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103.
- Bethke, P.C.; Jones, B. Apoplastic Synthesis of Nitric Oxide by Plant Tissues. Plant Cell. 2004, 16, 332–341.
- Moreau, M.; Lindermayr, C.; Durner, J.; Klessig, D.F. NO synthesis and signaling in plants—Where do we stand? Physiol. Plant. 2010, 38, 372–383.
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38.
- Alber, N.A.; Sivanesan, H.; Vanlerberghe, G.C. The occurrence and control of nitric oxide generation by the plant mitochondrial electron transport chain. Plant Cell Environ. 2017, 40, 1074–1085.
- Gupta, K.J.; Igamberdiev, A.U.; Manjunatha, G.; Segu, S.; Moran, J.F.; Neelawarne, B.; Bauwe, H.; Kaiser, W.M. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci. 2011, 181, 520–526.
- Tewari, R.K.; Prommer, J.; Watanabe, M. Endogenous nitric oxide generation in protoplast chloroplasts. Plant Cell Rep. 2013, 32, 31–44.
- Praveen, K.; Kumar, T.R.; Nand, S.P. Sodium nitroprusside-mediated alleviation of iron deficiency and modulation of antioxidant responses in maize plants. Aob Plants 2010, 2010, plq002.
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047.
- Grozeff, G.; Alegre, M.L.; Senn, M.E.; Chaves, A.R.; Bartoli, C.G. Combination of nitric oxide and 1-MCP on postharvest life of the blueberry (Vaccinium spp.) fruit. Postharvest Biol. Technol. 2017, 133, 72–80.
- Steelbeart, C.; Alegre, M.L.; Bahima, J.V.; Senn, M.E.; Grozeff, G. Nitric oxide improves the effect of 1-methylcyclopropene extending the tomato (Lycopersicum esculentum L.) fruit postharvest life. Sci. Hortic. 2019, 255, 193–201.
- Shi, K.K.; Liu, Z.C.; Wang, J.W.; Zhu, S.H.; Huang, D.D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611.
- Chaki, M.; Paz Morales, A.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647.
- Gonzalez-Gordo, S.; Bautista, R.; Claros, M.G.; Caas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570.
- Rodriguez-Ruiza, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2019, 12, 171–181.
- Cheng, G.P. Effect of Nitric Oxide on Ethylene Synthesis and Softening of Banana Fruit Slice during Ripening. J. Agric. Food Chem. 2009, 13, 5799–5804.
- Li, X.P.; Wu, B.; Guo, Q.; Wang, J.D.; Zhang, P.; Chen, W.X. Effects of Nitric Oxide on Postharvest Quality and Soluble Sugar Content in Papaya Fruit during Ripening. J. Food Process. Preserv. 2014, 38, 1–9.
- Hao, Y.Q.; Chen, F.H.; Wu, G.B.; Gao, W.Y. Impact of Postharvest Nitric Oxide Treatment on Lignin Biosynthesis-Related Genes in Wax Apple (Syzygium samarangense) Fruit. J. Agric. Food Chem. 2016, 64, 8483–8490.
- Yang, Y.; Zheng, Y.Y.; Liu, C.; Chen, L.; Ma, J.F.; Sheng, J.P.; Shen, L. Inhibition of nitric oxide synthesis delayed mature-green tomato fruits ripening induced by inhibition of ethylene. Sci. Hortic. 2016, 211, 95–101.
- Zhu, L.Q.; Du, H.Y.; Wang, W.; Zhang, W.; Shen, Y.G.; Wan, C.P.; Chen, J.Y. Synergistic effect of nitric oxide with hydrogen sulfide on inhibition of ripening and softening of peach fruits during storage. Sci. Hortic. 2019, 256, 108591.
- Qi, X.H.; Ji, Z.J.; Lin, C.; Li, S.F.; Liu, J.; Kan, J.; Zhang, M.; Jin, C.H.; Qian, C.L. Nitric oxide alleviates lignification and softening of water bamboo ( zizania latifolia ) shoots during postharvest storage. Food Chem. 2020, 332, 127416.
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266.
- Deng, L.L.; Pan, X.Q.; Chen, L.; Lin, S.; Sheng, J.P. Effects of preharvest nitric oxide treatment on ethylene biosynthesis and soluble sugars metabolism in ‘Golden Delicious’ apples. Postharvest Biol. Technol. 2013, 84, 9–15.
- Zhu, S.H.; Jie, Z. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 2007, 100, 1517–1522.
- Wu, F.H.; Yang, H.; Chang, Y.; Cheng, J.; Bai, S.; Yin, J. Effects of nitric oxide on reactive oxygen species and antioxidant capacity in Chinese Bayberry during storage. Sci. Hortic. 2012, 135, 106–111.
- De Paepe, A.; Van Der Straeten, D. Ethylene biosynthesis and signaling: An overview. Vitam. Horm. 2005, 72, 399–430.
- Manjunatha, G.; Lokesh, V.; Neelwarne, B. Nitric oxide in fruit ripening: Trends and opportunities. Biotechnol. Adv. 2010, 28, 489–499.
- Rafael, Z.; Marta, R.; Patricia, J.; Grazieli, B.P.; Sonia, C.S.A.; Claudia, M.F.; Eduardo, P.; Jose, M.P.; Corpas, F.J.; Magdalena, R.; et al. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. J. Exp. Bot. 2020, 3, 3.
- Zhu, S.; Liu, M.; Zhou, J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol. Technol. 2006, 42, 41–48.
- Ye, J.B.; Chen, F.H.; Wu, G.B. Effect of nitric oxide on physiology and quality of postharvest wax apple(Syzygium samarangense Merr et Perry) Fruits. J. Jim Univ. 2012, 17, 180–185.
- Wang, B.; Li, Z.; Han, Z.; Xue, S.; Prusky, D. Effects of nitric oxide treatment on lignin biosynthesis and texture properties at wound sites of muskmelons. Food Chem. 2021, 362, 130193.
- Yang, H.; Zhou, C.; Wu, F.; Cheng, J. Effect of nitric oxide on browning and lignification of peeled bamboo shoots. Postharvest Biol. Technol. 2010, 57, 72–76.
- Zhang, S.Q. Effects of nitric oxide on post-harvest physiology and quality of green asparagus. Chin. Agric. Sci. Bull. 2010, 26, 77–81.
- Ren, Y.F.; He, J.; Liu, H.; Liu, G.; Ren, X. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong’ mango fruit during ripening. Hortic. Environ. Biotechnol. 2017, 58, 27–37.
- Zheng, X.; Hu, B.; Song, L.; Jie, P.; Liu, M. Changes in quality and defense resistance of kiwifruit in response to nitric oxide treatment during storage at room temperature. Sci. Hortic. 2017, 222, 187–192.
