Spread of coronavirus disease 2019 (COVID-19) has significantly impacted the public health and economic sectors. It is urgently necessary to develop rapid, convenient, and cost-effective point-of-care testing (POCT) technologies for the early diagnosis and control of the plague’s transmission. Developing POCT methods and related devices is critical for achieving point-of-care diagnosis. The POCT devices based on microfluidic technology on the market, including paper-based microfluidic, centrifugal microfluidic, optical fluid, and digital microfluidic platforms.
- microfluidic
- COVID-19
- molecular assays
1. Introduction
2. Microfluidic Platform
2.1. Passive Microfluidic Platform—μPADs
Microfluidic paper-based analytical devices (µPADs) are a representative passive microfluidic platform, which use patterned paper as the carrier and distributes liquid through capillarity. μPADs are suitable for multiplex assays of various samples and are compatible with various assays. In contrast to traditional lateral flow strips, μPADs can autonomously drive multistep sequences, while the timing of the reagent’s movement can be controlled by adjusting the length and fluid volume of each channel [18]. Compared to traditional microfluidic labs, using paper sheets as a carrier also has unique advantages. Firstly, paper sheets have good biocompatibility, easy absorption of reagents, and are suitable for clinical analysis. Secondly, the inherent white background of paper sheets can provide contrast for color-based methods. Thirdly, paper is an ideal material for manufacturing environmentally friendly portable equipment because of its lightweight and low cost. Based on these advantages, μPADs have received attention in POCT applications, with rapid POCT detection of infectious diseases, especially in resource-poor areas, being one of the most popular areas. Herein, the following will discuss the development of μPADs for COVID-19 POCT.
2.2. Active Microfluidic Platforms
2.2.1. Centrifugal Microfluidic Platform
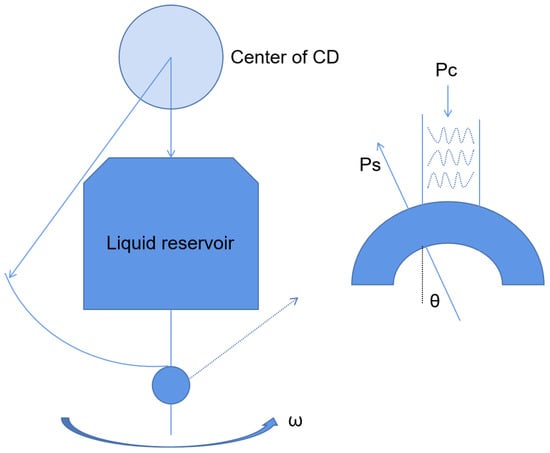
2.2.2. Optical Fluid Microfluidic Platform
2.2.3. Digital Microfluidics Platform
This entry is adapted from the peer-reviewed paper 10.3390/bios13020163
References
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220.
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228.
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 31 October 2022).
- Backer, J.A.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020, 25, 2000062.
- Wang, D.; Hu, B.; Hu, C. Transposed Data in a Table. JAMA—J. Am. Med. Assoc. 2021, 325, 1113.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Zhang, J.; Cai, Y.; Xiao, T.; Lu, J.; Peng, H.; Sterling, S.M.; Walsh, R.M., Jr.; Rits-Volloch, S.; Zhu, H.; Woosley, A.N.; et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science 2021, 372, 525–530.
- Weigl, J. Challenges in infectious disease control and the current pandemic by skewed distributions. Pravent. Gesundh. 2020, 15, 97–101.
- Nie, K.X.; Wang, C.; Li, X.W. Success of Big Infectious Disease Reimbursement Policy in China. Inq. J. Health Care Organ. Provis. Financ. 2020, 57, 46958020907788.
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA—J. Am. Med. Assoc. 2020, 323, 1239–1242.
- Deng, S.Q.; Peng, H.J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575.
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490.
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092.
- Jarrom, D.; Elston, L.; Washington, J.; Prettyjohns, M.; Cann, K.; Myles, S.; Groves, P. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: A rapid systematic review. BMJ Evid. Based Med. 2022, 27, 33–45.
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imohl, M.; Kleines, M. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J. Virol. Methods 2021, 288, 114024.
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imohl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394.
- Shrivastava, S.; Trung, T.Q.; Lee, N.E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866.
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020, 39, 107442.
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach. Biosens. Bioelectron. 2021, 177, 112672.
- Yin, K.; Ding, X.; Li, Z.; Sfeir, M.M.; Ballesteros, E.; Liu, C. Autonomous lab-on-paper for multiplexed, CRISPR-based diagnostics of SARS-CoV-2. Lab Chip 2021, 21, 2730–2737.
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; von Stetten, F. Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid. Nanofluidics 2011, 10, 1279–1288.
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758–1773.
- Malic, L.; Brassard, D.; Da Fonte, D.; Nassif, C.; Mounier, M.; Ponton, A.; Geissler, M.; Shiu, M.; Morton, K.J.; Veres, T. Automated sample-to-answer centrifugal microfluidic system for rapid molecular diagnostics of SARS-CoV-2. Lab Chip 2022, 22, 3157–3171.
- Dignan, L.M.; Woolf, M.S.; Tomley, C.J.; Nauman, A.Q.; Landers, J.P. Multiplexed Centrifugal Microfluidic System for Dynamic Solid-Phase Purification of Polynucleic Acids Direct from Buccal Swabs. Anal. Chem. 2021, 93, 7300–7309.
- Sampad, M.J.N.; Amin, M.N.; Hawkins, A.R.; Schmidt, H. FPGA Integrated Optofluidic Biosensor for Real-Time Single Biomarker Analysis. IEEE Photonics J. 2022, 14, 3701806.
- Ho, K.-L.; Liao, H.-Y.; Liu, H.M.; Lu, Y.-W.; Yeh, P.-K.; Chang, J.Y.; Fan, S.-K. Digital Microfluidic qPCR Cartridge for SARS-CoV-2 Detection. Micromachines 2022, 13, 196.
