Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Covalent organic frameworks (COFs) are a class of extended crystalline porous polymers that possess unique architectures with high surface areas, long-range order, and permanent porosity. It is known that the possible radioactive iodine species in the environment are iodate (IO3−), molecular iodine (I2), and organic iodine species (e.g., methyl iodide (CH3I) and ethyl iodide (CH3CH2I)). Different iodine species need to be handled in different ways.
- covalent organic frameworks
- mechanisms
- electron-rich groups
1. Introduction
As one of the clean energy sources that is most likely to replace fossil fuels, nuclear energy plays an extremely important role in many countries [1][2]. However, the utilization of nuclear energy faces a major problem related to the safe disposal of nuclear waste containing radioactive substances, especially radioactive iodine, which is difficult to handle in the actual environment due to its volatility, strong fluidity, and fast diffusion [3][4]. The main radioisotopes for iodine are 129I and 131I. 129I is considered to be one of the most dangerous byproducts in nuclear waste due to its long radioactive half-life (1.57 × 107 years) and negative effects on human metabolic processes (it can be accumulated in the human thyroid gland, causing serious diseases) and the environment. Additionally, although 131I has a short half-life (about 8 days), it is often combined with other hydrocarbons to yield organic compounds, such as methane iodide, which also make it extremely harmful to its ecological surroundings and human health [5][6][7]. If handled improperly, it will seriously restrict the development and application of nuclear energy. On the other hand, radioactive iodine has important applications in the medical field. For example, 125I seed implantation is widely applied in clinical brachytherapy, and 131I can be used for the examination of thyroid function and the treatment of malignant tumors [8]. In this regard, it is urgent to develop a highly efficient method to capture and store radioactive iodine.
It is known that the possible radioactive iodine species in the environment are iodate (IO3−), molecular iodine (I2), and organic iodine species (e.g., methyl iodide (CH3I) and ethyl iodide (CH3CH2I)). Different iodine species need to be handled in different ways [9][10][11]. Among them, volatile molecular iodine (I2) is the main chemical form of radioiodine in fission, which is of major concern due to its chemical and biological toxicities. Given this, developing functional adsorbents to efficiently capture radioiodine vapor is extremely significant. The traditional adsorbents developed for radioactive molecular iodine capture and storage are mainly inorganic adsorbents, such as zeolites, Ag-doped silica aerogels, clay, and activated carbon [12][13][14][15][16]. However, these inorganic adsorbents normally have low efficiency, high cost, instability toward moisture, or demanding application scenarios. For instance, the theoretical and practical adsorption capacities of Ag-doped silica aerogel are 1.18 g·g−1 and 0.10–0.31 g·g−1, respectively, and are far from meeting actual requirements [17]. For the elimination of radioiodine in complicated conditions, it is necessary to design novel materials with high sorption capacity, high stability, high selectivity, and low cost.
In recent years, the application of metal—organic frameworks (MOFs) and porous organic polymers (POPs) for iodine capture has attracted great interest. MOFs have been widely studied as adsorbents for molecular iodine because of their high surface area [18][19][20][21][22][23][24][25]. Yet, the stability of MOFs at high temperatures and in solution are generally poor, which limits their practical application. POPs, including hypercrosslinked polymers (HCPs) [26][27][28], conjugated microporous polymers (CMPs) [29][30][31][32], porous aromatic frameworks (PAFs) [33][34][35], and covalent organic frameworks (COFs) [36], are another type of porous material with higher stability that are connected via strong covalent bonds. They have been found to have a high potential for capturing and storing iodine, and many of them have achieved very high iodine capacities. As a unique class of POPs, COFs are distinguished from other POPs by their highly ordered internal structures and crystallinities, as well as their advantages of easy functionalization, low density, large BET surface area, intrinsic porosity, and superior chemical/thermal stability [36][37][38][39]. The fascinating features of COFs, with their atomically precise integration of scaffolds into 2D/3D topologies, have shown outstanding applications in many fields, such as gas adsorption, sensing, energy conversation, catalysis, etc. Since COF-1 and COF-5 were reported by Yaghi’s group [40] for the first time in 2005, COF materials have become a research focus of the current scientific and technological frontier. Several reviews have summarized the synthesis, characterization, and application of COFs [41][42][43][44][45][46]. However, there is still lack of reviews systematically focusing on the COFs applied in the iodine capture area. In 2017, Zhao et al. first applied COFs in the field of iodine capture and achieved remarkable results [47]. They reported a heteropore 2D COF (SIOC-COF-7), which showed an I2 adsorption capacity of 4.81 g·g−1 due to its large inner cavities, and a special structure of porous shells. This 2D COF also showed good adsorption performance towards dissolved I2 in the solution phase. Since then, many successful examples of COFs constructed by various monomers and functional building blocks (Scheme 1) have been reported and applied in the iodine capture area in recent years. These COFs, with their specific pore environments and tunable chemistry, can be easily functionalized to acquire effective iodine capture active sites. Although research on the application of COFs in iodine capture is still in its infancy, the unique structural characteristics of these materials, such as their tunable pore size, large surface area, and high crystallinity, make them highly competitive candidates for iodine capture applications.
Scheme 1. Various monomers and functional building blocks (amine groups and aldehyde groups) used for the construction of COFs with iodine capture performance. A—aldehyde; M—amine.
2. Mechanism of Iodine Capture by COFs
2.1. Methods for Studying Adsorption Mechanism
The mechanism of the iodine capture process by COFs mainly consists of physical adsorption, chemical adsorption, and a combination of physical and chemical adsorption. Detailed research on the adsorption mechanism could deepen our understanding of the adsorption process, and thus, promote the theoretical development of the design of COF-based materials with iodine-adsorption properties. In order to explore the adsorption mechanism, samples of COFs loaded with iodine (expressed as I2@COF) are usually studied via FT-IR spectra, Raman spectra, X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR), PXRD patterns, transmission electron microscopy (TEM), density functional theory (DFT) calculations, etc. The results of all analyses should be consistent with each other.
The fT-IR spectra of the original samples and those after the loading of iodine were compared. If the characteristic peak positions shift obviously or gradually decrease, or even disappear, it can be proven that there is chemical adsorption caused by the charge-transfer interaction between iodine and electron-rich groups. It can also enable us to infer which structural parts of COFs are related to the adsorption of I2. For example, as shown in Figure 1a, compared with the FT-IR spectra of PA-TT COF, the stretching vibration peak of the -C=N- of I2@PA-TT COF shows a significant shift from 1656 cm−1 to 1627 cm−1, indicating the existence of a chemisorption process caused by the charge-transfer interaction between iodine and the N atom of C=N [48].
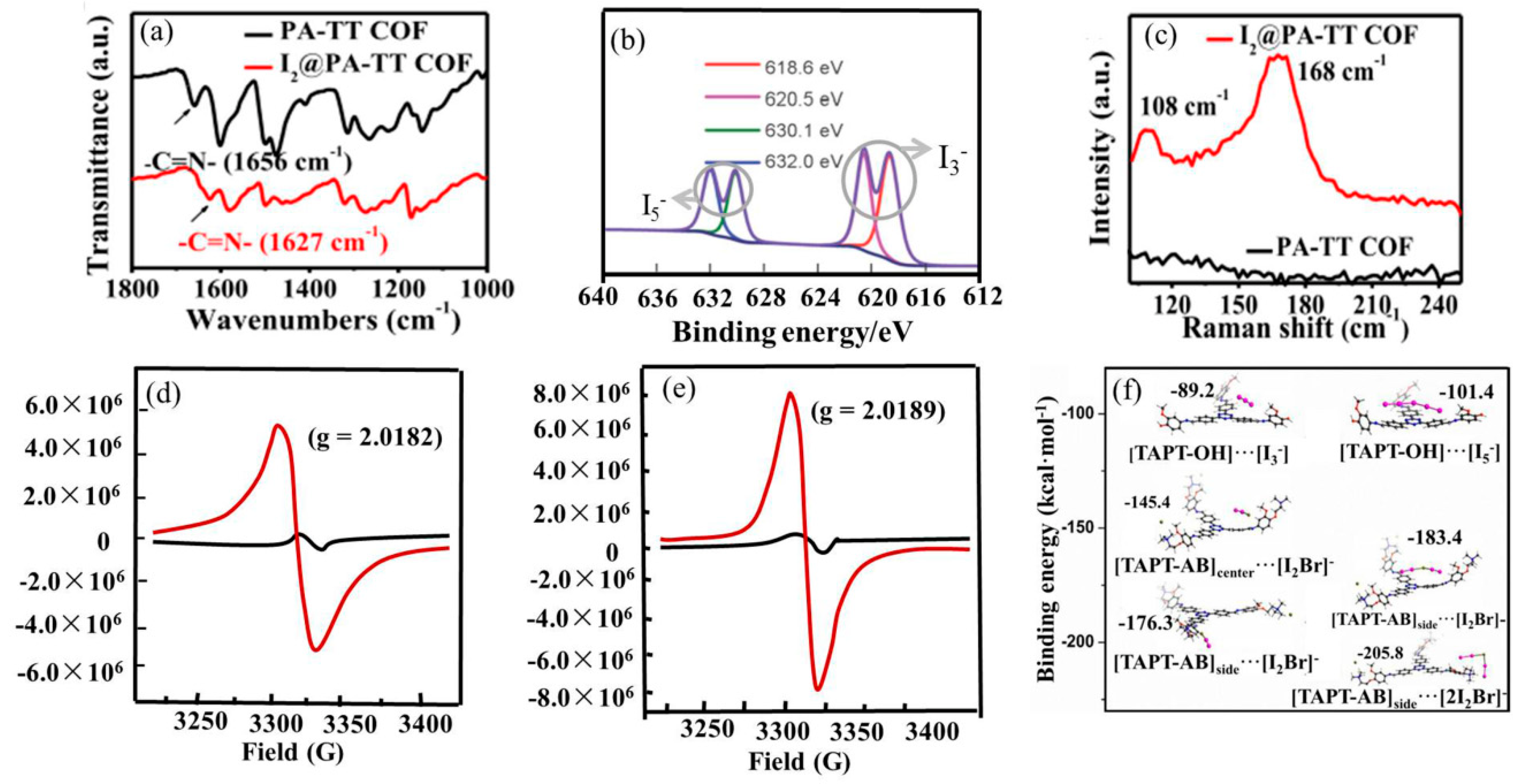
Figure 1. (a) FT-IR spectra of PA-TT COF and I2 @PA-TT COF. Reprinted/adapted with permission from Ref. [48]. Copyright (2023), Elsevier. (b) Iodine high-resolution XPS spectra of I2 @TTF-TD-COF. Reprinted/adapted with permission from Ref. [49]. Copyright (2022), Springer-Verlag GmbH. (c) Raman spectra of PA-TT COF and I2 @PA-TTCOF. Reprinted/adapted with permission from Ref. [48]. Copyright (2023), Elsevier. EPR spectra of JUC-560 (d) and JUC-561 (e) before (black curve) and after (red curve) iodine uptake. Reprinted/adapted with permission from Ref. [50]. Copyright (2021), Royal Society of Chemistry. (f) Density functional theory (DFT) calculations of the binding energies of TAPT-OH with I3− and I5−, and TAPT-AB with [I2Br]− and [2I2Br]− located at different positions. Reprinted with permission from Ref. [51]. Copyright (2021), John Wiley and Sons.
X-ray photoelectron spectroscopy (XPS) was conducted to investigate the existing state of iodine captured in COFs (such as I3− and I5−). Before iodine adsorption, the COFs exhibit no obvious peaks. After adsorption, strong peaks appear in the range of 617~620 eV and 629~632 eV for I3− and I5−, respectively (Figure 1b). So, it can be deduced that the absorbed iodine species in COFs existed as polyiodide anions.
Raman spectra were also used to reveal the chemical state of iodine inside the pores of COFs. No obvious peaks are found in COFs before the uptake of iodine. The intense peaks at around 107~109 cm−1 and 167~170 cm−1 emerge for I2@COFs, which can be attributed to the I3− and I5− ions, respectively (Figure 1c). If the peaks of I2, I3−, and I5− can be observed simultaneously, it indicates that elemental iodine and polyiodide anions co-existed in the channels of COFs and the iodine adsorption process was a combination of physisorption and chemisorption. XPS and Raman spectroscopy are the two most effective methods for revealing the chemical state of iodine within the pores of COFs.
The generation of radical cations after iodine adsorption was confirmed via electron paramagnetic resonance (EPR) studies. The original samples show a very weak EPR signal, while there is an approximate increase of two orders of magnitude in paramagnetic intensity after I2 doping. For example, the EPR studies on JUC-560 and JUC-561 show obvious peaks at g = 2.0182 and 2.0189, respectively (Figure 1d,e), clearly indicating the presence of TTF·+ radical cations oxidized by iodine [50].
Density functional theory (DFT) calculations can help researchers gain insight into how the COF frameworks bind iodine. It is well known that the binding energy can remarkably affect iodine capture. Normally, the stronger the interaction between iodine and the adsorption sites, the higher the iodine uptake. The binding energy between the model compounds was calculated via DFT and the corresponding conclusions were obtained by comparing the values. Han’s group [51] calculated the binding energies of the model molecules TAPT-OH and TAPT-AB with the identified iodine species. As shown in Figure 1f, the binding energies between TAPT-AB and [I2Br]−/[2I2Br]− are approximately twice that between TAPT-OH and I3−/I5−, suggesting a stronger affinity of TAPT-AB toward the iodine species. This is consistent with expectations and explains the experimental observations.
PXRD and transmission electron microscopy (TEM) analysis can show whether the crystallinity and morphology of COFs can be maintained throughout the iodine capture process.
2.2. Physical Adsorption of Iodine by COFs
Physical adsorption mainly depends on the surface areas, pore sizes and pore volumes of COFs. Thus, the functionalized architectures are designed to have high specific surface areas, and large pore sizes and pore volumes for efficient physisorption of iodine. For example, Liu et al. designed and synthesized four 2D COFs with different pore sizes for volatile iodine adsorption [52]. Finally, they revealed that the iodine uptake capacity was not only determined by the pore volume but also significantly affected by the intrinsic pore size.
Jiang’ group reported a series of 2D COFs with 1D open channels, which possessed various topologies from hexagonal to tetragonal and trigonal, and were free of specific binding sites and interchannel interpenetration [53]. So, the possibility of charge transfer was excluded. Among these 2D COFs, a TPB-DMTP-COF with a pore size of 3.3 nm and a pore volume of 1.28 cm3·g−1 achieved a remarkable iodine adsorption capacity (6.26 g·g−1) only in physical adsorption, which was driven by van der Waals forces. They proved that I2 capture does not require specific functionalization of the porous skeleton, and that pores of any shape or size can be 100% occupied by physical adsorption. However, its adsorption kinetics were quite slow (0.13 g·g−1·h−1).
2.3. Synergistic Effect of Physical and Chemical Adsorption
As aforementioned, porosity plays an important role in the adsorption of iodine vapor. However, in addition to the amount of adsorption, the adsorption rate is also a significant factor that must be considered. As for the TPB-DMTP-COF, it has an ultrahigh uptake capacity but takes 100 h to reach saturation, which is particularly unfavorable in the case of emergency disasters. Studying only physical adsorption—whereby the iodine uptake capacity of COF materials is determined by pore volume, while the iodine uptake kinetics are determined by pore connectivity and size—limits the application of COFs for iodine capture. The combination of physical and chemical adsorption is of paramount importance in the designed synthesis of COF materials for the capture of radioiodine. It is commonly believed that the interaction of I2 with electron-rich groups (that is, the so-called active sites, such as C=N, -NH2, triazine, pyridine, aromatic rings, etc., which can effectively adsorb electron-deficient I2 via the formation of charge-transfer complexes) and pore channels results in a combination of physical and chemical adsorption for the I2 capture process. The iodine vapor adsorbed into the pores through physical adsorption can readily generate the polyiodide species I3− and I5− due to the strong interactions between the exposed electron-rich groups and I2, implying that a chemisorption process occurs. In the meantime, iodine molecules may fill the remaining pore channels through physical adsorption.
At present, most COFs for iodine capture are based on the above principles. Zhao and co-workers prepared a 2D COF (COF-PA) containing quinoline and phenylacetylene units via post-functionalization of the TPB-DMTP-COF (A3-M10, Figure 2) [54]. XPS and Raman spectra prove that electrons are transferred from electron-rich quinoline units to electron-deficient iodine. They can not only form complex electron-deficient I2 via an electron-rich quinoline unit, but can also adsorb I2 via a chemical reaction with phenylacetylene moieties. Although the surface area and pore size of COF-PA were reduced compared with before functionalization, the adsorption rate was accelerated and the adsorption capacity was still high at low iodine concentrations. Very recently, Zhai et al. discovered two rare cationic COFs, C-TP-PDA-COFs and C-TP-BPDA-COFs (A5-M2, A5-M13), via a post-function process. The cationic C-TP-BPDA-COF exhibits a higher iodine capture value (6.11 g·g−1) than that of neutral COF [55].
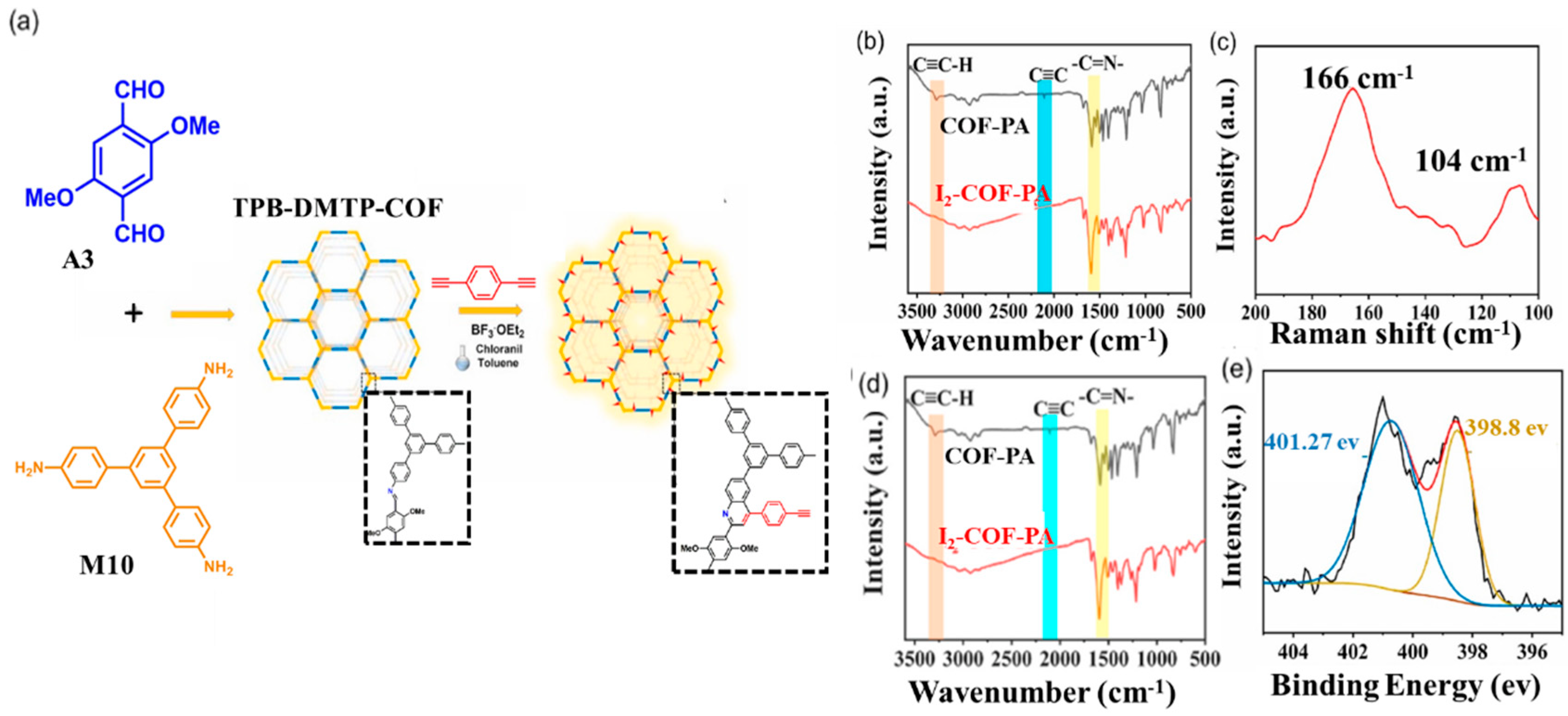
Figure 2. (a) Synthetic route. (b) FT-IR spectra. (c) Raman spectra of I2@COF-PA. (d) FT-IR spectra of COF-PA and I2@COF-PA. (e) XPS survey spectrum. Reprinted/adapted with permission from Ref. [54]. Copyright (2021), Elsevier.
The mechanism study found that after the adsorption of iodine by COF, before ionization, only the peak of the neutral iodine molecule existed in the XPS spectra, which suggests typical physical adsorption. However, after ionization, signals for both neutral I2 and anion I3− signals were observed. This indicates the presence of both physical and chemical adsorption. Chang et.al reported that two tetrathiafulvalene (TTF)-based COFs, JUC-560 (A16-M4) and JUC-561(A16-M8), achieve excellent iodine adsorption capacity (8.19 g·g−1) and ultrafast adsorption kinetics (0.70 g·g−1·h−1) [50]. These architectures are designed to have large specific surface areas for high iodine uptake through the physical process, and plentiful TTF functional groups for chemisorption. The synergistic effects of the physisorption and chemisorption processes together contributed to the superior iodine vapor adsorption capacities of the studied COF materials.
The above description indicates that the integration of the virtues of physical and chemical adsorption is of paramount importance in the designed synthesis of porous materials for the very challenging capture of radioiodine.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27249045
References
- Ewing, R.C.; Hippel, F.N.V. Nuclear waste management in the United States—Starting over. Science 2009, 325, 151–152.
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard. Mater. 2016, 318, 266–281.
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626.
- Haefner, D.R.; Tranter, T.J. Methods of Gas Phase Capture of Iodine from Fuel Reprocessing Off-Gas: A Literature Survey; Idaho National Laboratory: Idaho Falls, ID, USA, 2007.
- Mushkacheva, G.; Rabinovich, E.; Privalov, V.; Povolotskaya, S.; Shorokhova, V.; Sokolova, S.; Turdakova, V.; Ryzhova, E.; Hall, P.; Schneider, A.B.; et al. Thyroid abnormalities associated with protracted childhood exposure to 131I from atmospheric emissions from the Mayak weapons facility in Russia. Radiat. Res. 2006, 166, 715–722.
- Hoevea, J.E.T.; Jacobson, M.Z. Worldwide health effects of the Fukushima Daiichi nuclear accident. Energy Environ. Sci. 2012, 5, 8743–8757.
- Taylor, D.M. The radiotoxicology of iodine. J. Radioanal. Chem. 1981, 65, 195–208.
- Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; Wexler, J.A.; et al. Radiation Safety in the Treatment of Patients with Thyroid Diseases by Radioiodine 131I: Practice Recommendations of the American Thyroid Association. Thyroid 2011, 21, 335–346.
- Shimamoto, Y.S.; Takahashi, Y.; Terada, Y. Formation of Organic Iodine Supplied as Iodide in a Soil–Water System in Chiba, Japan. Environ. Sci. Technol. 2011, 45, 2086–2092.
- Hu, Q.; Zhao, P.; Moran, J.E.; Seaman, J.C. Sorption and transport of iodine species in sediments from the Savannah River and Hanford Sites. J. Contam. Hydrol. 2005, 78, 185–205.
- Yamaguchi, N.; Nakano, M.; Takamatsu, R.; Tanida, H. Inorganic iodine incorporation into soil organic matter: Evidence from iodine K-edge X-ray absorption near-edge structure. J. Environ. Radioact. 2010, 101, 451–457.
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O–Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181.
- Subrahmanyam, K.S.; Malliakas, C.D.; Sarma, D.; Armatas, G.S.; Wu, J.; Kanatzidis, M.G. Ion-exchangeable molybdenum sulfide porous chalcogel: Gas adsorption and capture of iodine and mercury. J. Am. Chem. Soc. 2015, 137, 13943–13948.
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Choi, D.Y.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062.
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899.
- Deuber, H. Investigations on the Retention of Elemental Radioiodine by Activated Carbons at High Temperatures. Nucl. Technol. 2017, 72, 44–48.
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326.
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal-organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595.
- Sava, D.F.; Rodriguez, M.A.; Chapman, K.W.; Chupas, P.J.; Greathouse, J.A.; Crozier, P.S.; Nenoff, T.M. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401.
- Sava, D.F.; Chapman, K.W.; Rodriguez, M.A.; Greathouse, J.A.; Crozier, P.S.; Zhao, H.; Chupas, P.J.; Nenoff, T.M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596.
- Zhang, X.; Silva, I.d.; Godfrey, H.G.W.; Callear, S.K.; Sapchenko, S.A.; Cheng, Y.; Vitórica-Yrezábal, I.; Frogley, M.D.; Cinque, G.; Tang, C.C.; et al. Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 16289–16296.
- Brunet, G.; Safin, D.A.; Aghaji, M.Z.; Robeyns, K.; Korobkov, I.; Woo, T.K.; Murugesu, M. Stepwise crystallographic visualization of dynamic guest binding in a nanoporous framework. Chem. Sci. 2017, 8, 3171–3177.
- Tang, Y.; Huang, H.; Li, J.; Xue, W.; Zhong, C. IL-induced formation of dynamic complex iodide anions in composites for efficient iodine capture. J. Mater. Chem. A 2019, 7, 18324–18329.
- Zhao, Q.; Zhu, L.; Lin, G.; Chen, G.; Liu, B.; Zhang, L.; Duan, T.; Lei, J. Controllable Synthesis of Porous Composite Beads for Iodine Capture. ACS Appl. Mater. Interfaces 2019, 11, 4263–42645.
- Chen, P.; He, X.; Pang, M.; Dong, X.; Zhao, S.; Zhang, W. Iodine Capture Using Zr-Based Metal—Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 20429–20439.
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683.
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231.
- McKeown, N.B.; Budd, P.M. Exploitation of Intrinsic Microporosity in Polymer-Based Materials. Macromolecules 2010, 43, 5163–5176.
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031.
- Zhuang, X.; Gehrig, D.; Forler, N.; Liang, H.; Wagner, M.; Hansen, M.R.; Laquai, F.; Zhang, F.; Feng, X. Conjugated Microporous Polymers with Dimensionality-Controlled Heterostructures for Green Energy Devices. Adv. Mater. 2015, 27, 3789–3796.
- Gu, C.; Huang, N.; Gao, J.; Xu, F.; Xu, Y.; Jiang, D. Controlled Synthesis of Conjugated Microporous Polymer Films: Versatile Platforms for Highly Sensitive and Label-Free Chemo- and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 4850–4855.
- Geng, T.; Zhang, W.; Zhu, Z.; Kai, X. Triazine-based conjugated microporous polymers constructing triphenylamine and its derivatives with nitrogen as core for iodine adsorption and fluorescence sensing I2. Micropor. Mesopor. Mat. 2019, 273, 163–170.
- Li, B.; Zhang, Y.; Krishna, R.; Yao, K.; Hang, Y.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Space, B.; et al. Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane. J. Am. Chem. Soc. 2014, 136, 8654–8660.
- Wu, X.; Shaibani, M.; Smith, S.J.D.; Konstas, K.; Hill, M.R.; Wang, H.; Zhang, K.; Xie, Z. Microporous carbon from fullerene impregnated porous aromatic frameworks for improving the desalination performance of thin film composite forward osmosis membranes. J. Mater. Chem. A. 2018, 6, 11327–11336.
- Ren, H.; Ben, T.; Sun, F.; Guo, M.; Jing, X.; Ma, H.; Cai, K.; Qiu, S.; Zhu, G. Synthesis of a porous aromatic framework for adsorbing organic pollutants application. J. Mater. Chem. 2011, 21, 10348–10353.
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
- Jin, E.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M.A.; Brady, M.A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q.; et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 2017, 357, 673–676.
- Baldwin, L.A.; Crowe, J.W.; Pyles, D.A.; McGrier, P.L. Metalation of a Mesoporous Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 15134–15137.
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic Covalent Organic Frameworks with Spiroborate Linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741.
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170.
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913.
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735.
- Sahoo, R.; Mondal, S.; Pal, S.C.; Mukherjee, D.; Das, M.C. Covalent-Organic Frameworks (COFs) as Proton Conductors. Adv. Energy Mater. 2021, 11, 2102300.
- Wang, J.L.; Zhuang, S.T. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046.
- Cai, Y.; Ling, Q.; Yi, Y.; Chen, Z.; Yang, H.; Hu, B.; Liang, L.; Wang, X. Application of covalent organic frameworks in environmental pollution management. Appl. Catal. A Gen. 2022, 643, 118733.
- Li, Y.; Song, X.; Zhang, G.; Wang, L.; Liu, Y.; Chen, W.; Chen, L. 2D Covalent Organic Frameworks toward Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2022, 15, e202200901.
- Yin, Z.-J.; Xu, S.-Q.; Zhan, T.-G.; Qi, Q.-Y.; Wu, Z.-Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269.
- Yan, X.; Yang, Y.X.; Li, G.R.; Zhang, J.H.; He, Y.; Wang, R.; Lin, Z.; Cai, Z.W. Thiophene-based covalent organic frameworks for highly efficient iodine capture. Chinese Chem. Lett. 2023, 34, 107201.
- Wang, G.; Xie, K.; Zhu, F.; Kan, J.; Li, S.; Geng, Y.; Dong, Y. Construction of Tetrathiafulvalene-based Covalent Organic Frameworks for Superior Iodine Capture. Chem. Res. Chinese Univ. 2022, 38, 409–414.
- Chang, J.H.; Li, H.; Zhao, J.; Guan, X.Y.; Li, C.M.; Yu, G.T.; Valtchev, V.; Yan, Y.S.; Qiu, S.L.; Fang, Q.R. Tetrathiafulvalene-based covalent organic frameworks for ultrahigh iodine capture. Chem. Sci. 2021, 12, 8452–8457.
- Xie, Y.Q.; Pan, T.T.; Lei, Q.; Chen, C.L.; Dong, X.L.; Yuan, Y.Y.; Shen, J.; Cai, Y.C.; Zhou, C.H.; Pinnau, I.; et al. Ionic Functionalization of Multivariate Covalent Organic Frameworks to Achieve an Exceptionally High Iodine-Capture Capacity. Angew. Chem. Int. Ed. 2021, 60, 22432–22440.
- An, S.; Zhu, X.; He, Y.; Yang, L.; Wang, H.; Jin, S.; Hu, J.; Liu, H. Porosity Modulation in Two-Dimensional Covalent Organic Frameworks Leads to Enhanced Iodine Adsorption Performance. Ind. Eng. Chem. Res. 2019, 58, 10495–10502.
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991.
- Zhao, Y.; Liu, X.; Li, Y.; Xia, M.; Xia, T.; Sun, H.; Sui, Z.; Hu, X.-M.; Chen, Q. Ultra-stable fluorescent 2D covalent organic framework for rapid adsorption and selective detection of radioiodine. Micropor. Mesopor. Mat. 2021, 319, 111046.
- Zhai, L.; Sun, S.; Chen, P.; Zhang, Y.; Sun, Q.; Xu, Q.; Wu, Y.; Nie, R.; Li, Z.; Mi, L. Constructing cationic covalent organic frameworks by a post-function process for an exceptional iodine capture via electrostatic interactions. Mater. Chem. Front. 2021, 5, 5463–5470.
This entry is offline, you can click here to edit this entry!
