Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Trimethylamine N-oxide (TMAO) is a metabolite produced by the gut microbiota and has been mainly associated with an increased incidence of cardiovascular diseases (CVDs) in humans. TMAO is a biomolecule capable of providing relevant information on the metabolic and immunological state of the human body. Having been linked to the pathogenesis and progression of several diseases, it could be a potential biomarker for diagnosis, prognosis, and therapeutic intervention.
- TMAO
- microbiota
- cardiovascular diseases
- neurological diseases
- metabolic diseases
- COVID-19
1. Kidney Disorders
Although 95% of TMAO is normally eliminated by the kidneys, this filtering function is affected by chronic kidney disease. Interestingly, TMAO was found to be a marker of survival in patients with chronic kidney disease in a prospective study using the glomerular filtration rate, C-reactive protein, and cystatin C as its evaluation parameters [1].
TMAO’s role in chronic kidney disease has also been examined in animal models. For instance, a decrease in the plasma concentration of TMAO attenuated the progression of chronic kidney disease [2]. In addition, TMAO has been reported to accelerate the development of diabetic kidney disease, exacerbating renal dysfunction and fibrosis by activating the NLRP3 inflammasome and by promoting the release of IL-1β and IL-18 [3]. Similarly, renal failure and inflammatory cell infiltration were exacerbated by TMAO treatment in CDK rats, leading the authors to conclude that this microbial metabolite made inflammation more robust. The cytokines MCP-1, TNF, IL-6, IL-1β, and IL-18 were activated through the p38 pathway. Oxidative stress was increased through the upregulation of NOX-4, the downregulation of SOX, and the activation of the NLRP3 inflammasome by caspase-1 and IL-1β [4].
In addition, research has been performed on patients with chronic kidney disease. The TMAO level was higher in patients with chronic kidney disease than in the control group, and it was highest in patients with declining renal function. In detail, the concentration of TMAO correlated negatively with the glomerular filtration rate and positively with the levels of IL-6 and fibrinogen. Moreover, an elevated TMAO level was associated with a drop in the 5-year survival and with a 6.3-fold increase in the risk of mortality [5].
The level of TMAO being inversely correlated with the glomerular filtration rate explained the higher level. Moreover, the FMO3-induced production of TMAO may be another factor contributing to the increased TMAO level. The renal elimination of TMAO was only slightly affected by tubular secretion and reabsorption, suggesting that the goal of reducing the TMAO level must be achieved by targeting its production [6].
Overall, the aforementioned reports provide the basis for proposing TMAO as a therapeutic target. However, more research is needed on whether a decreased plasma concentration of TMAO influences the glomerular filtration rate. In a double-blind randomized study, an evaluation was performed on the use of probiotics for lowering the concentration of TMAO and for improving kidney function, finding no significant difference with the placebo group [7]. Therefore, it is necessary to investigate the long-term effect of probiotics together with dietary intervention as a strategy for diminishing the amount of TMAO and for preserving renal function.
2. Metabolic Syndrome
Metabolic syndrome (MS) involves at least three of the following five conditions: abdominal obesity, systemic arterial hypertension, insulin resistance, a high serum concentration of triglycerides, and a low serum concentration of HDLs (high-density lipoproteins). MS is a risk factor for the development of type 2 diabetes and cerebrovascular and cardiovascular diseases [8]. The presence of a high serum concentration of TMAO has been associated with metabolic syndrome, probably due to the multiple pathways of inflammation in which this metabolite participates, including the accumulation of fatty deposits in the blood vessels and tissues, which generates a fatty liver, visceral obesity, and atherosclerosis [9].
According to the results of a transversal study, a high serum concentration of TMAO (≥8.74 µM) was proposed as a biomarker for MS, as it implies a high risk of developing this disorder [10]. Additionally, more details about the diseases related to metabolic syndrome are mentioned in the following sections.
3. Obesity
Obesity is a worldwide public health problem and is defined as excessive fat accumulation in the body. There has been a 5-fold increase in the number of obesity cases since 1997 [11]. Obesity is a risk factor for several chronic noncommunicable diseases, including cardiovascular diseases and diabetes [12].
An elevated TMAO level is linked to obesity and a vitamin D deficiency, although the causality has not been established [13]. Surely the diet is an important factor in the connection between TMAO and obesity. In a random clinical trial of 62 participants with overweight and obesity factors, 3 groups were formed and observed over 8 weeks: a control group, a cod diet group, and a salmon diet group. The level of TMAO was higher in the cod diet group than the salmon diet or control groups [14].
TMAO accumulation has been proven to be unaffected in the short run by other foods, such as eggs (in postmenopausal women) or those containing flavonoids (in obese patients) [15][16]. In a study on obese adults, the level of TMAO was lower in participants assigned exercise in combination with a hypocaloric diet (<500 kcal) compared to those with an equicaloric diet. When developing interventions that seek to decrease the level of TMAO in overweight or obese patients, it is needed to consider the bone mineral loss of the spine, which is reportedly independent of changes in body weight [17]. A systematic review found a nonlinear relationship between the TMAO level and the body mass index (BMI) among 56,556 apparently healthy patients [18].
Finally, the mechanisms of TMAO in obesity progression have been examined in animal models. The deletion of FMO3, the enzyme responsible for the conversion of TMA into TMAO, conferred protection against obesity in mice by allowing for the begging of white adipose tissue [19].
4. Diabetes
Diabetes is a chronic metabolic disease characterized by an elevated blood glucose level that is capable, over time, of leading to damage to the heart, blood vessels, eyes, and kidneys. The most common form of this disease in adults is type 2 diabetes, which begins with insulin resistance and may progress to a low insulin level. In the last 30 years, the incidence of diabetes has been growing rapidly around the world [20].
As was mentioned before, TMAO can induce ER stress through PERK and FoxO1 activation, leading to the expression of INSR and insulin cellular resistance and elevating the risk of developing type 2 diabetes [21]. There are several clinic studies that confirm the association between TMAO and type 2 diabetes. In one study, the serum concentration of TMAO was higher in T2D patients compared to those with prediabetes or without diabetes [22].
In the POUND LOST trial, a minor reduction in the TMAO level was linked to small improvements in the blood concentration of glucose and insulin as well as in the degree of insulin resistance (the latter was evaluated with the homeostatic model assessment, HOMA) in participants who consumed a high-fat hypocaloric diet [23]. There was a relationship between TMAO, T2D, and its complications. It has been hypothesized that microvascular endothelial damage is produced by both the disease and by TMAO [24][25].
5. Metabolic-Dysfunction-Associated Fatty Liver Disease
Metabolic-dysfunction-associated fatty liver disease (MAFLD), previously denominated nonalcoholic fatty liver disease, is an inflammatory condition that is closely linked to the level of TMAO as well as to MS, T2D, and obesity. There is a close relationship between adipose tissue dysfunction (characterized by increased cytokine/chemokine production as well as an influx of CD4+ macrophages, CD8+ T cells, dendritic cells, and natural killer cells) and insulin resistance in the liver, muscle, and adipose tissues [26]. MAFLD should be promptly identified because it usually progresses to more serious forms, including steatohepatitis, steatofibrosis, cirrhosis, and cancer [27]. It is also associated with the development of liver cancer as well as esophageal, gastric, uterine, colon, breast, and other cancers [28]. Indeed, MAFLD is linked to a greater risk of mortality from any cause [29].
The relationship between TMAO and the progression from MAFLD to steatohepatitis has mainly been described in obese patients with T2D [30]. This relationship owes itself to the capacity of TMAO to promote de novo lipogenesis in the liver, which increases the generation of bile acid and inhibits farnesoid X receptor activation [31]. Hence, TMAO has been proposed as a possible therapeutic target for MAFLD [32].
6. Systemic Arterial Hypertension
Systemic arterial hypertension (SAH) is highly prevalent in adults and represents the main risk factor for cardiovascular and cerebrovascular diseases. In the respective patients, an elevated serum TMAO concentration is associated with an atherosclerotic effect and an overabundance of proinflammatory cytokines [33]. In animal models, a high level of TMAO prolonged the hypertensive response to angiotensin II, which increases vasoconstriction and the concentration of the intracellular ionic calcium in the arterioles [34]. It also caused a greater synthesis of vasopressin, expression of acuaporin-2, and osmotic pressure [35].
In mice, the TMAO concentration was associated with age and was positively correlated with a higher systolic blood pressure (SBP) and arterial stiffness independently of other cardiovascular risk factors. An elevated TMAO level is associated with more abundant amount of advanced glycation end products in the aorta, and in ex vivo experiments, it boosted the SBP and exacerbated arterial stiffness via the glycation end products and oxidative stress [36]. In another animal model, the treatment of obstructive-sleep-apnea-induced hypertension in rats with the probiotic Lactobacillus rhamnosus GG strain provided a lower level of TMAO and CD4+ T cell induced-type I inflammation, leading to a reduction in hypertension [37].
7. Vascular Diseases
The effects of an elevated TMAO level have most commonly been analyzed in relation to cardiovascular and cerebrovascular diseases. TMAO has been evaluated as a biomarker of major adverse cardiovascular events (MACEs), cardiac insufficiency, and mortality by vascular disease [38][39][40]. The mechanism of TMAO in cardiovascular diseases is shown in Figure 1.
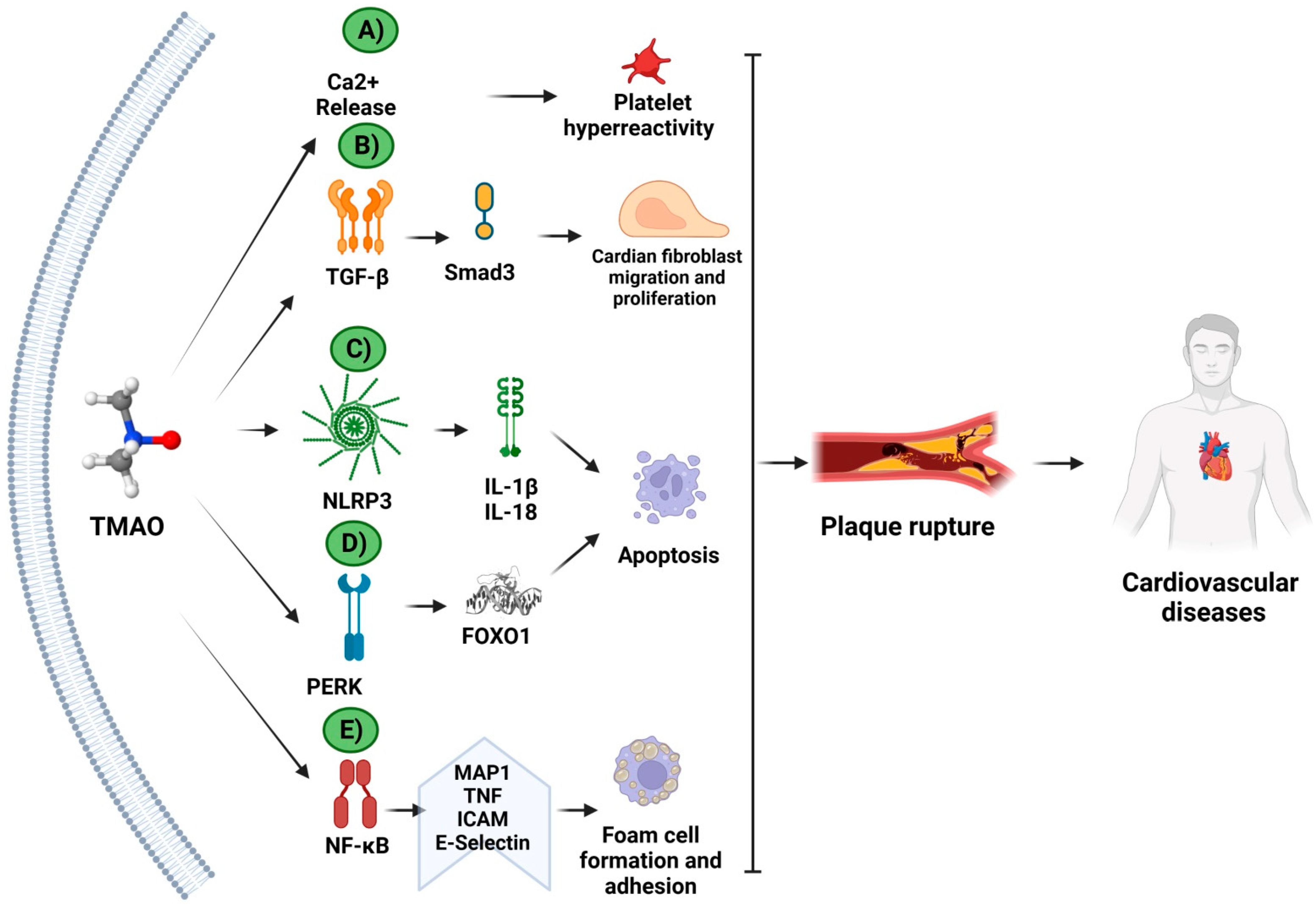
Figure 1. Molecular mechanisms of TMAO in cardiovascular diseases. The mechanism of TMAO in cardiovascular diseases has been studied in animal and/or cellular models. TMAO increases the (A) Ca2+ release and platelet hyperactivity; (B) TFG-β activation and Smad3 signaling pathway; (C) NLRP3 inflammasome activation, inducing apoptosis; (D) PERK-unfolding protein response (UPR) activation and endoplasmic reticulum stress; and (E) NF-κB translocation and foam cell formation and adhesion. Together, these mechanisms contribute to plaque ruptures, and, therefore, to heart disease. (Created with BioRender.com).
Medical interventions have been utilized to evaluate a potential effect on the TMAO levels of patients. For instance, 100 mL of Sanhuang Xiexin (a traditional Chinese formula) was administered 2 times per day for 1 week to 121 patients with an acute ischemic cerebrovascular disease under standard treatment. This prospective observational study found that the level of TMAO was lower in 61 of the patients in the treated group than in those with no administration. In addition, the risk of ischemic events was also lower in the Sanhuang Xiexin group versus the control group during the third and sixth months [41].
TMAO activity in the development of cardiovascular diseases has been assessed in vitro. The exposure of the cardiac mitochondria of rats to TMAO impaired β-oxidation and decreased the pyruvate metabolism. Thus, an elevated TMAO level could be considered to be a risk factor for cardiovascular events due to the disturbance of the energy metabolism in the cardiac tissue [42].
Probiotics were consumed by male patients with stable coronary artery disease for six weeks, finding improved endothelial function and reduced inflammation, but the TMAO concentration remained the same [43]. In another attempt to diminish the level of TMAO, an autologous fecal transplant was performed on patients with metabolic syndrome using tissues from a vegan donor. This intervention resulted in changes in the composition of the gut microbiota but not in the level of any measured parameter related to vascular inflammation, including TMAO [44].
Finally the resveratrol is a natural polyphenol contained in grapes, berries, and other foods, and it is used for the treatment of several metabolic diseases, such as atherosclerosis [45]. Its consumption reportedly modulates the composition of the gut microbiota, increasing the Bacteroidetes–Firmicutes ratio and the growth of Bacteroides, Lactobacillus, and Bifidobacterium. When ApoE −/− mice (a murine model of atherosclerosis) were treated with choline and then resveratrol, TMAO synthesis was blocked. Since FMO3 production was upregulated, the effect of reducing the TMAO level did not occur in the liver due to FMO3 but rather due to the increase in the Bacteroides–Firmicutes ratio and the growth of Lactobacillus and Bifidobacterium, which was evidenced by a lower bacterial content in the ileum and the repression of the farnesoid X receptor. Thus, resveratrol attenuated TMAO-induced atherosclerosis by decreasing the TMAO levels and by modulating the gut microbiota composition [46].
Further research is needed on probiotics and other treatments aimed at diminishing the TMAO concentration and at lowering the risk factors associated with cardiovascular diseases.
8. Neurological Diseases
Recently, TMAO has been reported to exhibit neuroinflammatory activity. The proposed mechanism is shown in Figure 2.
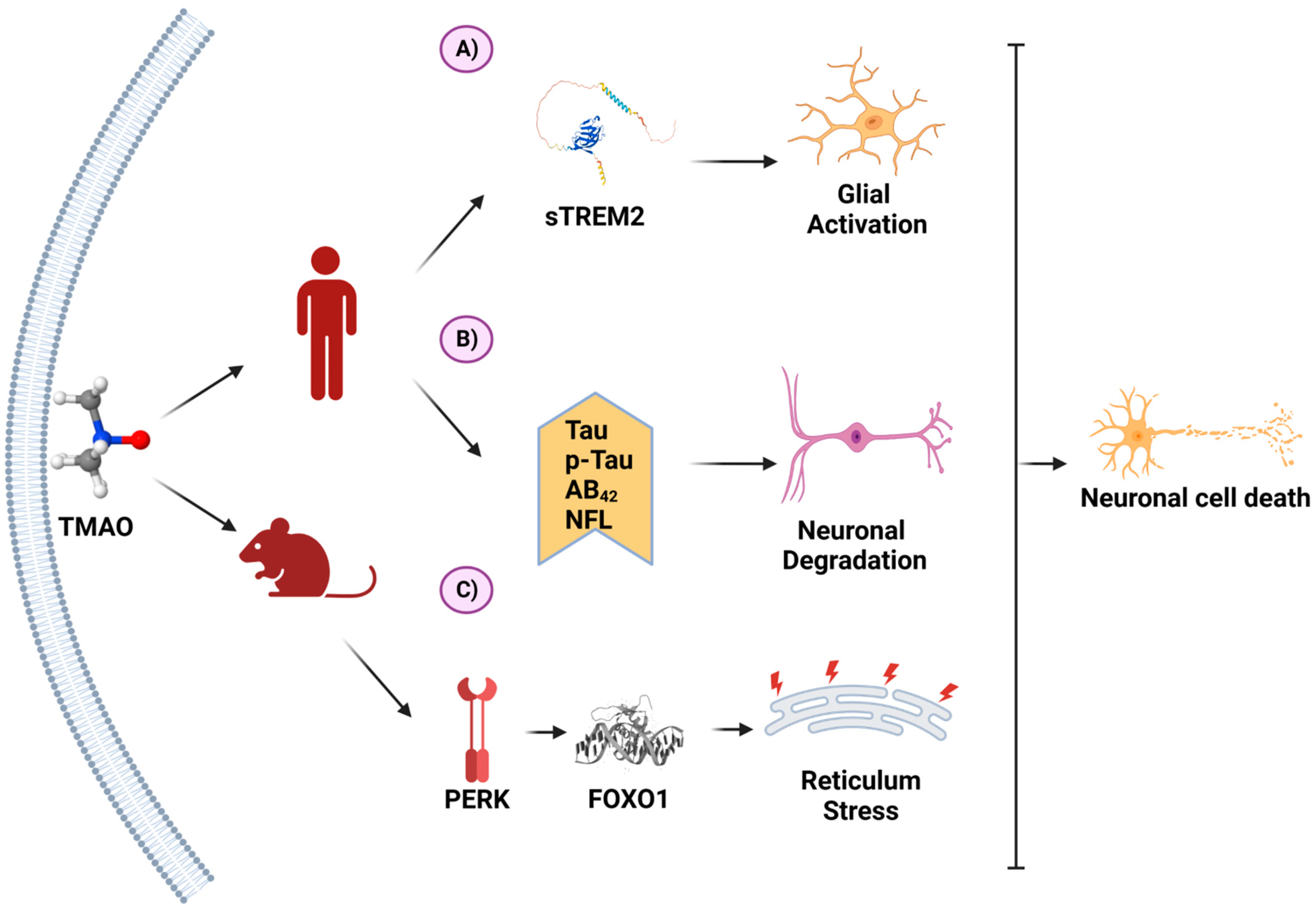
Figure 2. TMAO and its molecular mechanisms in neurons. (A) In a clinical study on patients with Alzheimer’s disease (AD), TMAO was positively associated with sTREM2, a biomarker for glial activation. (B) In another clinical study on AD, TMAO was associated with higher levels of AD biomarkers. (C) In an animal model of AD, TMAO induced the unfolding protein response through PERK activation, inducing endoplasmic reticulum stress. These processes favor neuron degeneration and death. (Created with BioRender.com).
A relationship was detected between the intake of red meat or cheese and the level of carnitine in human cerebrospinal fluid samples. Moreover, there was a positive correlation between TMAO and sTREM2, a glial activation marker [47].
In patients with acute ischemic stroke, high TMAO levels were linked to early neurological deterioration. The probable explanation is that several inflammatory markers, such as C-reactive protein and IL-6, are associated with the progression of neurological deficits. Since TMAO is able to trigger an exacerbated inflammatory response, it can likely cause the worsening of neurological deterioration in such patients [48].
The use of ApoE −/− mice to study the connection between TMAO and atherosclerosis was mentioned in the previous section on vascular diseases. ApoE is expressed by several cellular types but is mainly found in some immune cells of the central nervous system: hepatocytes, astrocytes, and microglia. In these cells, it plays an important role in the metabolism of plasma lipoproteins and in the growth and maturation of the neurons.
The apoE gene has been identified as a genetic risk factor for atherosclerosis, cardiovascular diseases, and Alzheimer’s disease (AD). The ε4 allele of the apoE gene increases the risk of Alzheimer’s disease, while the ε2 allele provides protection against it [49]. The carriers of the ε4 allele also have a higher risk of hypercholesterolemia and atherosclerosis because this allele leads to a greater plasma concentration of LDLs [50]. In addition, the ApoE protein interacts with the beta-amyloid peptide. The latter, one of the main elements in AD pathogenesis, is responsible for neural death and brain degeneration.
A critical factor of AD progression is an exaggerated inflammatory response (as is the case with atherosclerosis as well), and TMAO promotes inflammation and oxidative stress. Hence, an elevated level of this microbial metabolite could help to trigger the exacerbated inflammatory response that accompanies Alzheimer’s disease, an idea reinforced by the altered gut microbiota found in AD patients. Such an alteration is mainly manifested as a decreased abundance of Firmicutes and Bifidobacterium and an increased abundance of Bacteroidetes, which are genera linked to TMA production. The proportion of these genera in the microbiota composition is also modified in patients with cardiovascular diseases [51].
Given that inflammation plays an important role in Alzheimer’s disease, it is not surprising that several immune molecules have been linked to its pathogenesis, including TLR4, TGF-β, CXCR4, MCP-1, MIP2, Cox-2, NLRP3, PERK, and FoxO1. As previously stated, in vitro studies have shown that the aforementioned proteins are activated or induced by TMAO.
There are some reports on the level of TMAO in AD patients. In detail, a transversal study documented a higher level of TMAO in the cerebrospinal fluid of AD patients with mild cognitive deterioration than in that of those with no cognitive impairment. When comparing patients with severe dementia to those with a mild cognitive deterioration, higher TMAO amounts were exhibited by the former. Furthermore, TMAO was positively correlated with Alzheimer’s disease and with the biomarkers of neuronal degeneration (p-tau, p-tau/AB42, total tau, and neurofilament light chain protein) [52].
In animal models, an elevated level of TMAO has been positively correlated with more pronounced AD symptoms, such as long-term potentiation, increased neuronal loss, and altered spatial learning and memory [53]. Thus, AD patients might experience relief of their symptoms by means of interventions capable of modifying the concentration of TMAO in circulation, which could be accomplished in part by reducing the elements of the gut microbiota responsible for activating the TMAO pathway. The corresponding treatments would be expected to modulate the inflammatory response and to attenuate the neuronal deterioration of patients suffering from Alzheimer’s disease.
9. Cancer
In recent years, TMAO has been analyzed as a risk factor for cancer, especially colorectal cancer. Several studies have demonstrated that an altered microbiota composition is associated with colorectal cancer (CRC), which involves a greater abundance of the species of the genus Fusobacterium [54]. The degree of TMAO production depends on the composition of the gut microbiota.
The first report of a TMAO association with colorectal cancer was based on a genome-wide systemic analysis conducted in 2015, which established the genetic relationship between TMAO and several diseases, including cardiovascular diseases, metabolic syndrome, and some cancer types such as colorectal cancer [55].
Subsequently, research was carried out on patients to explore the possible link between TMAO and colorectal cancer. The first example was the Women’s Health Initiative Observational Study, finding a positive correlation between TMAO and female CRC patients. In the same study, the patients with higher TMAO levels showed a 1.9-fold greater risk of proximal tumors, a 2.3-fold greater risk of rectal tumors, and a 1.8-fold greater risk of local tumors. These risks were associated with a low level of vitamin B12 [56].
In another study performed on male patients in Finland, the alpha tocopherol and beta carotene cohorts showed no association between colorectal cancer and TMAO. Nevertheless, choline (one of the precursors of the TMAO biosynthesis pathway) was associated with colorectal cancer [57].
In China, TMAO concentrations were evaluated for 108 patients (68 men and 40 women), finding a significantly higher level in CRC patients than in the healthy controls. Regarding an elevated level of TMAO, there was a positive correlation with a more advanced CRC stage and with the presence of metastases as well as a negative correlation with disease-free survival. Hence, the results pointed to TMAO as a plausible prognostic marker for colorectal cancer [58].
By utilizing bioinformatics, TMAO has been linked to the development of other cancers, such as ovarian, breast, and gastric cancers. However, such TMAO activity has not been fully explored [55]. Besides colorectal cancer, only prostate cancer has been directly linked to TMAO [59].
Although the mechanisms linking TMAO to different cancers are not completely understood, they likely involve the proinflammatory potential of this microbial metabolite. As explained herein, TMAO is able to trigger an inflammatory response through several pathways, including the production of inflammatory markers regulated by NF-kB, Smad3, NLRP3, and the unfolded protein response in J774A macrophages [60][61][62][63].
It has also been proposed that TMAO increases the risk of cancer through oxidative stress, which damages DNA when antioxidant defenses are deficient. In addition, higher levels of reactive oxygen species are found in cancer versus healthy cells, thus contributing to the sustenance of the malignant cell phenotype [64]. Therefore, the TMAO ability to induce oxidative stress may favor the growth and proliferation of cancer cells, leading to cancer development or the worsening of its clinical symptoms.
Another risk factor where TMAO is involved is the induction of insulin resistance. Obesity, insulin resistance, and type 2 diabetes are risk factors of developing cancers such as endometrial, pancreatic, liver, and colorectal cancers [65]. They can be explained by changes in the expression of insulin receptors [66], and, as is mentioned in a previous section, these receptors can be induced by TMAO through PERK/FoxO1 activation. IRS-1 and IRS2 receptors are critical for colorectal cancer prognoses, where both receptors are associated with metastases [67][68].
10. COVID-19
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. It first appeared in China in 2019 and then spread rapidly around the world, creating a pandemic with millions of cases of infection and death. Although most people infected with the virus only experience mild respiratory symptoms and recover without medical intervention, the symptoms can be life-threatening and can require advanced medical care [69].
The risk factors for severe COVID-19 are underlying medical conditions such as cardiovascular diseases, diabetes, chronic respiratory diseases, and cancer. SARS-CoV-2 activates a stress pathway in the host immune system, promoting an aberrant response in the adaptive and innate immune systems and probably leading to pneumonia and death [70]. As aforementioned, TMAO has an impactful role in the pathogenesis of cardiovascular diseases and diabetes, which are known risk factors for severe COVID-19. Moreover, like COVID-19, TMAO is able to modulate the immune response. Hence, it has been suggested that TMAO contributes to the COVID-19 pathogenesis.
Evidence exists of an interaction between SARS-CoV-2 and the NLRP3 inflammasome which occurs to increase IL-1 and IL-18 production through NF-ҡB activation [71]. Some studies on SARS-CoV-2-infected patients pointed to a relationship between TMAO and the development of severe cases of COVID-19. For example, an alteration in the gut microbiota was reported in COVID-19 patients (compared to healthy subjects). Whereas the relative abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi was correlated with the COVID severity, Faecalibacterium prausnitzii correlated negatively with the same factor [72]. Both the Coprobacillus and Clostridium species contain the cutC gene, while the Clostridium species also bares the grdH gene according to the UNIPROT database (www.uniprot.org). Thus, it is highly probably that both Coprobacillus and Clostridium contribute to TMA production.
When TMAO and its precursors were measured in patients with COVID-19, a low level of betaine was associated with a poor outcome. Hence, this metabolite was proposed as a biomarker of the risk factors for COVID-19. However, no significant difference was detected between the TMAO levels of COVID-19 patients versus those of the healthy controls [73]. The study served as a proof of concept, meaning that more research is required to clarify the connection between TMAO and COVID-19.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11020431
References
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455.
- Zhang, W.; Miikeda, A.; Zuckerman, J.; Jia, X.; Charugundla, S.; Zhou, Z.; Kaczor-Urbanowicz, K.E.; Magyar, C.; Guo, F.; Wang, Z.; et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci. Rep. 2021, 11, 518.
- Fang, Q.; Zheng, B.; Liu, N.; Liu, J.; Liu, W.; Huang, X.; Zeng, X.; Chen, L.; Li, Z.; Ouyang, D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats With Diabetic Kidney Disease. Front. Physiol. 2021, 12, 682482.
- Lai, Y.; Tang, H.; Zhang, X.; Zhou, Z.; Zhou, M.; Hu, Z.; Zhu, F.; Zhang, L.; Nie, J. Trimethylamine-N-Oxide Aggravates Kidney Injury via Activation of p38/MAPK Signaling and Upregulation of HuR. Kidney Blood Press. Res. 2022, 47, 61–71.
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS ONE 2016, 11, e0141738.
- Pelletier, C.C.; Croyal, M.; Ene, L.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; Lemoine, S.; Guebre-Egziabher, F.; Juillard, L.; Soulage, C.O. Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins 2019, 11, 635.
- Borges, N.A.; Stenvinkel, P.; Bergman, P.; Qureshi, A.R.; Lindholm, B.; Moraes, C.; Stockler-Pinto, M.B.; Mafra, D. Effects of Probiotic Supplementation on Trimethylamine-N-Oxide Plasma Levels in Hemodialysis Patients: A Pilot Study. Probiotics Antimicrob. Proteins 2019, 11, 648–654.
- Meigs, J. Metabolic Syndrome (Insulin Resistance Syndrome or Syndrome X). 2021. Available online: https://www.uptodate.com/contents/metabolic-syndrome-insulin-resistance-syndrome-or-syndrome-x?source=related_linkAccess (accessed on 16 June 2022).
- Croci, S.; D’Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites. Nutrients 2021, 13, 1389.
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971.
- WHO. Obesity and Overweight; WHO: Geneva, Switzerland, 2018.
- Berkey, C.S.; Rockett, H.R.H.; Field, A.E.; Gillman, M.W.; Frazier, A.L.; Camargo, C.A., Jr.; Colditz, G.A. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 2000, 105, E56.
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 1310.
- Hagen, I.V.; Helland, A.; Bratlie, M.; Midttun, Ø.; McCann, A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; Ueland, P.M.; Gudbrandsen, O.A. TMAO, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. Eur. J. Nutr. 2020, 59, 2249–2259.
- Zhou, T.; Heianza, Y.; Chen, Y.; Li, X.; Sun, D.; DiDonato, J.A.; Pei, X.; LeBoff, M.S.; Bray, G.A.; Sacks, F.M.; et al. Circulating Gut Microbiota Metabolite Trimethylamine N-Oxide (TMAO) and Changes in Bone Density in Response to Weight Loss Diets: The POUNDS Lost Trial. Diabetes Care 2019, 42, 1365–1371.
- Angiletta, C.J.; Griffin, L.E.; Steele, C.N.; Baer, D.J.; Novotny, J.A.; Davy, K.P.; Neilson, A.P. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Funct. 2018, 9, 5350–5361.
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41.
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. 2020, 21, e12993.
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461.
- WHO. Diabetes; WHO: Geneva, Switzerland, 2022.
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes 2016, 124, 251–256.
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: The POUNDS Lost trial. Gut 2019, 68, 263–270.
- Steinke, I.; Ghanei, N.; Govindarajulu, M.; Yoo, S.; Zhong, J.; Amin, R.H. Drug Discovery and Development of Novel Therapeutics for Inhibiting TMAO in Models of Atherosclerosis and Diabetes. Front. Physiol. 2020, 11, 567899.
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719.
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842.
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int. J. Mol. Sci. 2019, 20, 5037.
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236.
- Flores-Guerrero, J.L.; Post, A.; van Dijk, P.R.; Connelly, M.A.; Garcia, E.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Circulating trimethylamine-N-oxide is associated with all-cause mortality in subjects with nonalcoholic fatty liver disease. Liver Int. 2021, 41, 2371–2382.
- León-Mimila, P.; Villamil-Ramírez, H.; Li, X.S.; Shih, D.M.; Hui, S.T.; Ocampo-Medina, E.; López-Contreras, B.; Morán-Ramos, S.; Olivares-Arevalo, M.; Grandini-Rosales, P.; et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021, 47, 101183.
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, 1900257.
- Li, X.; Hong, J.; Wang, Y.; Pei, M.; Wang, L.; Gong, Z. Trimethylamine-N-Oxide Pathway: A Potential Target for the Treatment of MAFLD. Front. Mol. Biosci. 2021, 8, 733507.
- Zhang, W.-Q.; Wang, Y.-J.; Zhang, A.; Ding, Y.-J.; Zhang, X.-N.; Jia, Q.-J.; Zhu, Y.-P.; Li, Y.-Y.; Lv, S.-C.; Zhang, J.-P. TMA/TMAO in Hypertension: Novel Horizons and Potential Therapies. J. Cardiovasc. Transl. Res. 2021, 14, 1117–1124.
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115.
- Liu, M.; Han, Q.; Yang, J. Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2019, 41, 312–322.
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure With Aging in Mice and Humans. Hypertension 2021, 78, 499–511.
- Liu, J.; Li, T.; Wu, H.; Shi, H.; Bai, J.; Zhao, W.; Jiang, D.; Jiang, X. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4+ T cell induced-type I inflammation. Biomed. Pharmacother. 2019, 112, 108580.
- Gao, J.; Yan, K.-T.; Wang, J.-X.; Dou, J.; Wang, J.; Ren, M.; Ma, J.; Zhang, X.; Liu, Y. Gut microbial taxa as potential predictive biomarkers for acute coronary syndrome and post-STEMI cardiovascular events. Sci. Rep. 2020, 10, 2639.
- Zhou, X.; Jin, M.; Liu, L.; Yu, Z.; Lu, X.; Zhang, H. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. Esc Heart Fail. 2020, 7, 189–194.
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut Microbiome and Plasma Microbiome-Related Metabolites in Patients With Decompensated and Compensated Heart Failure. Circ. J. 2019, 83, 182–192.
- Song, J.; Chen, X.; Lyu, Y.; Zhuang, W.; Zhang, J.; Gao, L.; Tong, X. Sanhuang Xiexin decoction promotes good functional outcome in acute ischemic stroke. Brain Behav. 2019, 9, e01185.
- Makrecka-Kuka, M.; Volska, K.; Antone, U.; Vilskersts, R.; Grinberga, S.; Bandere, D.; Liepinsh, E.; Dambrova, M. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol. Lett. 2017, 267, 32–38.
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102.
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7, e008342.
- Chung, J.H.; Manganiello, V.; Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554.
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15.
- Thorstenson, J.C.; Heston, M.B.; Zarbock, K.R.; Carlsson, C.M.; Engelman, C.D.; Deming, Y.; Johnson, S.C.; Ulland, T.K.; Asthana, S.; Kollmorgen, G.; et al. Diet and APOE as moderators of the relationship between trimethylamine N-oxide and biomarkers of Alzheimer’s disease and glial activation. Alzheimer’s Dement. 2021, 17, e051827.
- Hou, L.; Zhang, Y.; Zheng, D.; Shi, H.; Zou, C.; Zhang, H.; Lu, Z.; Du, H. Increasing trimethylamine N-oxide levels as a predictor of early neurological deterioration in patients with acute ischemic stroke. Neurol. Res. 2020, 42, 153–158.
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312.
- Mahley, R.W.; Rall, S.C., Jr. Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genom. Hum. Genet. 2000, 1, 507–537.
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537.
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124.
- Gao, Q.; Wang, Y.; Wang, X.; Fu, S.; Zhang, X.; Wang, R.-T. Decreased levels of circulating trimethylamine N-oxide alleviate cognitive and pathological deterioration in transgenic mice: A potential therapeutic approach for Alzheimer’s disease. Aging 2019, 11, 8642–8663.
- Zhou, Z.; Chen, J.; Yao, H.; Hu, H. Fusobacterium and Colorectal Cancer. Front. Oncol. 2018, 8, 371.
- Xu, R.; Wang, Q.; Li, L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015, 16 (Suppl. S7), S4.
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.Y.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452.
- Guertin, K.A.; Li, X.S.; Graubard, B.I.; Albanes, D.; Weinstein, S.J.; Goedert, J.J.; Wang, Z.; Hazen, S.L.; Sinha, R. Serum Trimethylamine N-oxide, Carnitine, Choline, and Betaine in Relation to Colorectal Cancer Risk in the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 945–952.
- Liu, X.; Liu, H.; Yuan, C.; Zhang, Y.; Wang, W.; Hu, S.; Liu, L.; Wang, Y. Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark. Med. 2017, 11, 443–447.
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.L.; Zeisel, S.H.; Willett, W.C.; Chan, J.M. Choline intake and risk of lethal prostate cancer: Incidence and survival. Am. J. Clin. Nutr. 2012, 96, 855–863.
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357.
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70.
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767.
- Mohammadi, A.; Gholamhoseyniannajar, A.; Yaghoobi, M.M.; Jahani, Y.; Vahabzadeh, Z. Expression levels of heat shock protein 60 and glucose-regulated protein 78 in response to trimethylamine-N-oxide treatment in murine macrophage J774A.1 cell line. Cell. Mol. Biol. 2015, 61, 94–100.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197.
- Giovannucci, E.L.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685.
- Vella, V.; Milluzzo, A.; Scalisi, N.M.; Vigneri, P.; Sciacca, L. Insulin Receptor Isoforms in Cancer. Int. J. Mol. Sci. 2018, 19, 3615.
- Esposito, D.L.; Aru, F.; Lattanzio, R.; Morgano, A.; Abbondanza, M.; Malekzadeh, R.; Bishehsari, F.; Valanzano, R.; Russo, A.; Piantelli, M.; et al. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS ONE 2012, 7, e36190.
- Slattery, M.L.; Samowitz, W.; Curtin, K.; Ma, K.N.; Hoffman, M.; Caan, B.; Neuhausen, S. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1206–1214.
- WHO. COVID-19; WHO: Geneva, Switzerland, 2022.
- Terruzzi, I.; Senesi, P. Does intestinal dysbiosis contribute to an aberrant inflammatory response to severe acute respiratory syndrome coronavirus 2 in frail patients? Nutrition 2020, 79–80, 110996.
- Shi, C.-S.; Nabar, N.R.; Huang, N.-N.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019, 5, 101.
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.
- Israr, M.Z.; Ibrahim, W.; Salzano, A.; Sarmad, S.; Wilde, M.J.; Cordell, R.L.; Greening, N.J.; Brightling, C.E.; Siddiqui, S.; Suzuki, T. Association of gut-related metabolites with respiratory symptoms in COVID-19: A proof-of-concept study. Nutrition 2022, 96, 111585.
This entry is offline, you can click here to edit this entry!
