Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Obesity is a chronic metabolic complication, and its management requires long-term medication, lifestyle modifications, and dietary interventions. Patients taking anti-obesity medications may suffer from side effects such as psychiatric disorders, anxiety, depression, and vitamin deficiency. Various classes of natural compounds are promising agents to combat the obesity pandemic. Developing safer drugs may require polytherapeutic strategies to combat the global obesity pandemic.
- obesity
- metabolic syndrome
- synthetic drugs
- natural compounds
1. Introduction
Obesity, a metabolic complication, was initially considered a disease of positive energy balance due to overeating and a sedentary lifestyle. This perception led to the belief that dietary and short-term pharmacological interventions can easily control the obesity pandemic [1]. However, the scientific community in 1985 recognized obesity as a chronic disorder. The first approved class of drugs for obesity was amphetamines, which were subsequently removed from the market due to addiction and adverse events associated with the long-term use of amphetamines. This highlighted the need to design and develop safer alternatives for long-term use in obesity management [2]. The treatment of obesity is highly patient-centric, and various pharmacological and surgical approaches may require depending on the affected individuals. Effective strategies for treating obesity involve lifestyle intervention, complementary medicine, and alternative therapy, including drug treatment or bariatric surgery [1]. Thus, acupuncture is a good example of an effectively used alternative therapy in obesity coping due to its positive effect on hypothalamus functioning [3].
The pharmacological treatment for obesity becomes necessary given that the latest data from the World Obesity Federation suggests that 2.7 billion adults will fall under the obese category by 2025; thus, a huge demand for medical care and therapeutic interventions will arise in the near future. The rising global epidemic will result in huge medical costs pegged at USD 1.2 trillion per annum unless various interventions control the epidemic. Several anti-obesity drugs have been approved in the past. For instance, phentermine, diethylpropion, rimonabant, taranabant, sibutramine, orlistat, lorcaserin, and tesofensine are some of the anti-obesity medications for weight management [4]. Several anti-obesity drugs target monoamine neurotransmitter pathways, producing a feeling of fullness [5]. However, several anti-obesity drugs targeted towards neurotransmitters were withdrawn due to adverse psychiatric side effects and thus should not be prescribed for obese patients with psychological disorders [6]. Importantly, very limited behavioral data are available for existing anti-obesity drugs, making it essential to collect behavioral data during the early stages of drug development. Moreover, a lack of understanding of the molecular, cellular, and physiological targets of anti-obesity drugs may hamper the progress of drug development and consequently delay finding novel and effective anti-obesity molecules [7].
2. The Use of Natural Constituents in the Prevention and Treatment of Obesity
Herbal substances are regarded as an important target for drug development because of the wide variety of phytoconstituents and their few adverse effects [8]. A huge number of bioactive compounds from medicinal plants are beneficial in obesity coping. Among the secondary metabolites of plants, mainly polyphenols and terpenoids (Figure 1), they have demonstrated effective weight management properties [8][9][10]. Some of the alkaloids have good potential in the treatment of obesity, but the significant toxicity of most of them narrows the range of their application [11].
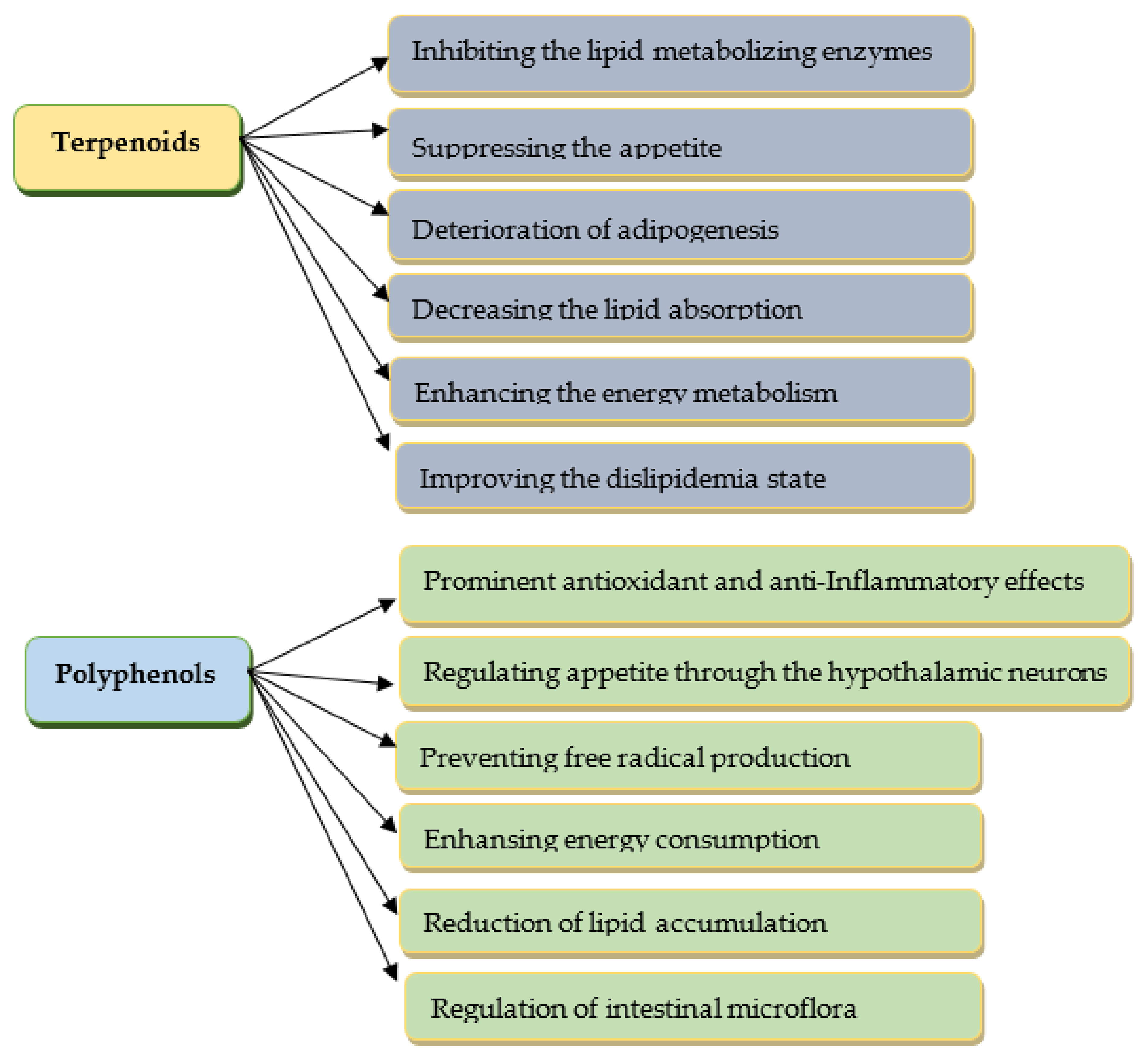
Figure 1. The anti-obesity effects of selected phytoconstituents.
The anti-obesity effects of phytoconstituents manifest in different ways: through inhibiting the lipid/carbohydrate-metabolizing enzymes, suppressing the appetite and adipogenesis, and inhibition of lipid absorption, as well as enhancing energy metabolism [9][12]. The modern “omics” technologies (genomics, transcriptomics, proteomics, and metabolomics) effectively evaluate the traditional healthcare phytosubstances as sources of new natural biocompounds as potential anti-obesity agents [13].
Recently, experimental research demonstrated that polyphenols as strong antioxidants were effective prebiotics in managing obesity induced by a high-fat diet [14]. As oxidative stress is crucial in the pathophysiology of obesity, modifying the concentration of inflammation mediators is associated with the number and size of adipocytes, lipogenesis, regulating appetite through the hypothalamic neurons, etc. [15]. Polyphenols could reduce body weight through different mechanisms [10][16][17]. Randomized controlled clinical trials conducted by Moorthy et al. [14] assessed the effect of polyphenols on body composition in the overweight, obese population. These studies showed some decrease in body weight by a mean of 1.47 ± 0.58 kg. Additionally, polyphenols could effectively prevent increases in weight [14]. The polyphenol-rich extract of the Vaccinium corymbosum leaves modified with arginine demonstrated its effectiveness in managing the metabolic syndrome [18]. Suzuki et al. [19] found a positive effect of black and green tea catechins on obesity. Quercetin and other flavonoids with antioxidant, anti-inflammatory, and hepatoprotective effects can effectively prevent metabolic diseases [20][21][22]. The intake of flavonoids can reduce the risk of metabolic syndrome disorders and rare side effects [20].
Nani et al. [23] concluded that the overproduction of reactive oxygen species is associated with the inflammatory process in obesity mediated through nuclear factor-κB. Chen et al. [24] reported that polyphenols could enhance the energy consumption and weight loss due to increased fat oxidation. Polyphenols are regarded as being very effective in the inactivation of reactive oxidant species [25]. Polyphenolic compounds from fruits and vegetables reduce lipid accumulation and enhance intestinal microflora [24]. The fruits and leaves of some Ericaceae species (Vaccinium corymbosum, Vaccinium myrtillus, etc.) [18][26][27] possess significant lipid-lowering properties and anti-obesity potential due to the presence of valuable phenolic compounds. Polyphenols of marine algae can transform problematic ‘white’ adipose tissue into ‘brown’ (rich in mitochondria) and, in this way, enhance energy consumption [28].
In the last decade, several researchers [29][30] have investigated the therapeutic effect of Ginkgo biloba extract in treating obesity and related disorders. The long-term therapy using an excerpt from Ginkgo biloba leaves showed an anti-obesogenic effect on rats [29]. Thomaz et al. [30] revealed that Ginkgo biloba extract mainly consists of flavonoids (25.21%). The chromatographic analysis revealed that flavonoids such as quercetin, kaempferol, rutin, and isorhamnetin were its predominant components [30].
The discovery of leptin at the end of the 20th century created hopes for an effective treatment of obesity, as this peptide hormone effectively regulates the body mass and lipolysis [31]. However, the development of resistance to the leptin influence, which is characterized by the overconsumption of nutrients due to reduced satiety, has been a big obstacle [32]. Liu et al. [32] discovered that the pentacyclic triterpene celastrol isolated from the roots of Tripterygium wilfordi possesses a significant anti-obesity effect as a leptin sensitizer. It can suppress food intake and causes up to 45% weight loss in obese mice by increasing the leptin sensitivity. In addition to the ability to regulate leptin sensitivity and lipid metabolism, it also positively influences the gut microbiota [33].
The anti-obesity potential of triterpene saponins was elucidated by Marrelli et al. [34]. Saponins can modulate adipogenesis and appetite and inhibit pancreatic lipase [34]. Alkaloid capsaicin, an active component of chili peppers, demonstrated great anti-obesity potency [35]. For decades, natural guanidine-containing phytosubstances from the French lilac (Galega officinalis) were used for their antidiabetic, hypolipidemic, and antiaging effects [36].
The weight management effect of carotenoids was found by Mounien et al. [37]. Gammone and D’Orazio [38] found the anti-obesity effect of fucoxanthin (carotenoid from marine algae). Carotenoid lycopene, which accumulates significantly in ripe tomatoes, demonstrated protection against diabetes and obesity [39]. Bjørklund et al. [40] revealed the ability of the other carotenoid astaxanthin, synthesized by numerous microalgae, yeasts, and bacteria, to manage the overweight outcome. Radice et al. [41] found that supplementation of the experimental animals with astaxanthin had positive effects on a variety of symptoms associated with obesity through the hypoglycemic and lipid-lowering capacity, as well as mitigating the immune system. Calanus oil, a natural product from marine crustacean Calanus finmarchicus, which is rich in astaxanthin, has a noticeable effect in treating low-grade inflammation related to obesity [42].
It should be noted that seaweeds were regarded as promising sources of various anti-obesity agents, such as phlorotannins, alginates, fucoxanthin, and fucoidans [12]. Fucoxanthin and fucoidans could inhibit lipid absorption and metabolism, as well as the differentiation of adipocytes, alginates reduce the feeling of hunger, and polyphenol phlorotannin possesses significant antioxidant and anti-inflammatory properties [12].
Cannabidiol from Cannabis sativa, which is widely known for its neurological effects, also has been considered an anti-inflammatory, antitumor, and anti-obesity agent [43]. As cannabinoid receptors regulate food consumption, thermogenesis, and inflammation, the intake of cannabinoids could help to reduce food intake and body weight [44].
Recently, De Blasio et al. [45] demonstrated that essential oils as multicomponent mixtures of volatile terpenoids and other bioactive compounds promote the decrease of fat mass and exert a positive weight management effect. It should be mentioned that essential oils exert these health-promoting effects when inhaled or taken with the diet [45]. Artemisinin, a sesquiterpenoid from the Artemisia annua, is a famous antimalarial drug [46]. In addition to the anti-parasite activity, artemisinin has also displayed antitumor, anti-inflammatory, and anti-obesity properties. Its anti-inflammatory and immunoregulatory effects are valuable in obesity coping, since chronic inflammation is implicated in the pathogenesis of metabolic disorders [42][46]. Islam et al. [47] summarized that several diterpenoids exert anti-obesity effects through various mechanisms. Among them, carnosol, carnosic acid, steviol, and andrographolide could be examples of effective weight management agents.
Experimental evidence was obtained of the anti-obesity effects of Ananas comosus juice [48] and papain (proteolytic enzyme) from Carica papaya fruits [49]. The anti-obesity properties of sulforaphane from broccoli (Brassica oleracea var. italica) were revealed by Ranaweera et al. [50].
Saffron (Crocus sativus) stigmas are famous spices and a promising natural antioxidant, anticancer, and anti-obesity phytosubstance [51][52]. Aromatic compound safranal and some carotenoids (crocin and picrocrocin) are regarded as the main bioactive constituents of Crocus sativus stigmas [52].
As it is known, many health disorders, such as diabetes, chronic inflammatory diseases, and obesity, are associated with uncontrolled sugar consumption [53]. As an artificial sweetener, Xylitol effectively prevents metabolic syndrome and obesity [53][54]. It can reduce the increased blood glucose level, body weight, and other unhealthy syndromes [53].
Some vitamins also possess substantial anti-obesity potential [55][56]. The antioxidant and hepatoprotective activity of tocopherol helps in preventing metabolic syndrome [57]. A deficiency of some vitamins in the body can cause excess weight. Thus, Thomas-Valdés et al. [55] concluded that most vitamins were deficient in obese persons, especially the fat-soluble vitamins, vitamin B12, folic acid, and ascorbic acid.
This entry is adapted from the peer-reviewed paper 10.3390/ph16020212
References
- Lu, X.; Jin, Y.; Li, D.; Zhang, J.; Han, J.; Li, Y. Multidisciplinary Progress in Obesity Research. Genes 2022, 13, 1772.
- Coulter, A.A.; Rebello, C.J.; Greenway, F.L. Centrally Acting Agents for Obesity: Past, Present, and Future. Drugs 2018, 78, 1113–1132.
- Wang, L.; Yu, C.C.; Li, J.; Tian, Q.; Du, Y.J. Mechanism of Action of Acupuncture in Obesity: A Perspective from the Hypothalamus. Front. Endocrinol. 2021, 12, 632324.
- Kang, J.; Park, C.-Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25.
- Kim, G.W.; Lin, J.E.; Blomain, E.S.; Waldman, S.A. Antiobesity pharmacotherapy: New drugs and emerging targets. Clin. Pharm. Ther. 2014, 95, 53–66.
- Moreira, F.A.; Crippa, J.A. The psychiatric side-effects of rimonabant. Braz. J. Psychiatry 2009, 31, 145–153.
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present, and future. Dis. Model. Mech. 2012, 5, 621–626.
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713.
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.A.K.; Chahal, J.; Dalal, S.; Kataria, S. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278.
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280.
- Yang, X.-D.; Ge, X.-C.; Jiang, S.-Y.; Yang, Y.-Y. Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front. Endocrinol. 2022, 13.
- Chu, W.-L.; Phang, S.-M. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222.
- Sahib, N.; Saari, N.A.I.; Khatib, A.; Mahomoodally, F.; Abdul Hamid, A. Plants’ Metabolites as Potential Antiobesity Agents. Sci. World J. 2012, 2012, 436039.
- Moorthy, M.; Sundralingam, U.; Palanisamy, U. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299.
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786.
- Farhat, G.; Drummond, S.; Al-Dujaili, E. Polyphenols and Their Role in Obesity Management: A Systematic Review of Randomized Clinical Trials. Phytother. Res. PTR 2017, 31, 1005–1018.
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid Med. Cell Longev. 2021, 2021, 3149223.
- Koshovyi, O.; Granica, S.; Piwowarski, J.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush Blueberry (Vaccinium corymbosum L.) Leaves Extract and Its Modified Arginine Preparation for the Management of Metabolic Syndrome-Chemical Analysis and Bioactivity in Rat Model. Nutrients 2021, 13, 2870.
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305.
- Gouveia, H.; Urquiza-Martinez, M.V.; Manhaes-de-Castro, R.; Costa-de-Santana, B.J.R.; Villarreal, J.P.; Mercado-Camargo, R.; Torner, L.; de Souza Aquino, J.; Toscano, A.E.; Guzman-Quevedo, O. Effects of the Treatment with Flavonoids on Metabolic Syndrome Components in Humans: A Systematic Review Focusing on Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 8344.
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364.
- Skakun, N.; Stepanova, N. Comparative evaluation of the hepatoprotective, antioxidant and choleretic activity of flavonoid drugs. Vrachebnoe Delo 1989, 52–54.
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985.
- Chen, L.; Pu, Y.; Xu, Y.; He, X.; Cao, J.; Ma, Y.; Jiang, W. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 157, 111202.
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. Biofactors 2022, 48, 255–273.
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138.
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of anti-obesity and lipid-lowering properties of Vaccinium myrtillus leaves powder extract in a hamster model. J. Basic Clin Physiol. Pharm. 2018, 29, 697–703.
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589.
- Hirata, B.K.S.; Cruz, M.M.; de Sa, R.; Farias, T.S.M.; Machado, M.M.F.; Bueno, A.A.; Alonso-Vale, M.I.C.; Telles, M.M. Potential Anti-obesogenic Effects of Ginkgo biloba Observed in Epididymal White Adipose Tissue of Obese Rats. Front. Endocrinol. 2019, 10, 284.
- Thomaz, F.M.; de Jesus Simao, J.; da Silva, V.S.; Machado, M.M.F.; Oyama, L.M.; Ribeiro, E.B.; Alonso Vale, M.I.C.; Telles, M.M. Ginkgo biloba Extract Stimulates Adipogenesis in 3T3-L1 Preadipocytes. Pharmaceuticals 2022, 15, 1294.
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887.
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011.
- Xu, S.; Feng, Y.; He, W.; Xu, W.; Xu, W.; Yang, H.; Li, X. Celastrol in metabolic diseases: Progress and application prospects. Pharm. Res. 2021, 167, 105572.
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404.
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37.
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164.
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562.
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214.
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharm. Res 2020, 159, 104966.
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167.
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of astaxanthin in animal models of obesity-associated diseases: A systematic review and meta-analysis. Free Radic. Biol Med. 2021, 171, 156–168.
- Gasmi, A.; Mujawdiya, P.K.; Shanaida, M.; Ongenae, A.; Lysiuk, R.; Dosa, M.D.; Tsal, O.; Piscopo, S.; Chirumbolo, S.; Bjørklund, G. Calanus oil in the treatment of obesity-related low-grade inflammation, insulin resistance, and atherosclerosis. Appl. Microbiol. Biotechnol. 2020, 104, 967–979.
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114.
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. J. Mol. Sci. 2018, 19, 5875.
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832.
- Shen, S.; Liao, Q.; Lyu, M.; Wong, Y.-K.; Zhang, X.; Zhou, J.; Ma, N.; Wang, J. The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol. Ther. 2020, 216, 107696.
- Islam, M.; Ali, E.; Mubarak, M. Anti-obesity effect of plant diterpenes and their derivatives: A review. Phytother. Res. 2020, 34.
- El-Shazly, S.A.; Ahmed, M.M.; Al-Harbi, M.S.; Alkafafy, M.E.; El-Sawy, H.B.; Amer, S.A.M. Physiological and molecular study on the anti-obesity effects of pineapple (Ananas comosus) juice in male Wistar rat. Food Sci. Biotechnol. 2018, 27, 1429–1438.
- Kang, Y.M.; Kang, H.A.; Cominguez, D.C.; Kim, S.H.; An, H.J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9983.
- Ranaweera, S.S.; Natraj, P.; Rajan, P.; Dayarathne, L.A.; Mihindukulasooriya, S.P.; Dinh, D.T.T.; Jee, Y.; Han, C.H. Anti-obesity effect of sulforaphane in broccoli leaf extract on 3T3-L1 adipocytes and ob/ob mice. J. Nutr. Biochem. 2022, 100, 108885.
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Mohd Yusof, B.N.; Noor, M.S. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308.
- Mykhailenko, O.; Petrikaite, V.; Korinek, M.; El-Shazly, M.; Chen, B.H.; Yen, C.H.; Hsieh, C.F.; Bezruk, I.; Dabrisiute, A.; Ivanauskas, L.; et al. Bio-guided bioactive profiling and HPLC-DAD fingerprinting of Ukrainian saffron (Crocus sativus stigmas): Moving from correlation toward causation. BMC Complement. Med. Ther. 2021, 21, 203.
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237.
- Pearlman, M.; Obert, J.; Casey, L. The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 1–8.
- Thomas-Valdés, S.; Vaz-Tostes, M.; Anunciação, P.; Silva, B.; Pinheiro-Sant’Ana, H. Association between Vitamin Deficiency and Metabolic Disorders Related to Obesity. Crit. Rev. Food Sci. Nutr. 2016, 57, 3332–3343.
- Wang, X.; Xu, M.; Li, Y. Adipose Tissue Aging and Metabolic Disorder, and the Impact of Nutritional Interventions. Nutrients 2022, 14, 3134.
- Gons’kyĭ, I.; Korda, M.; Klishch, I. Status of the free radical oxidation and antioxidant system in rats with toxic liver damage; effect of tocopherol and dimethylsulfoxide. Ukr. Biokhim. Zh. 1991, 63, 112–116.
This entry is offline, you can click here to edit this entry!
