You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
From the radiation protection point of view, less important natural radionuclides are 238U, 226Ra, 40K, and Th isotopes. However, 238U is a long-lived (t½ = 4.5 × 109 a) alpha emitter whose chemical toxicity exceeds its radiological toxicity. Although isotope 238U is not considered to cause significant radiation exposure, it is still the mother nuclide of a whole uranium decay series, producing isotopes of astatine, bismuth, lead, polonium, protactinium, radium, radon, thallium, and thorium while decaying. 40K also has a very long half-life (t½ = 1.28 × 109 a).
- natural radionuclides
- radioecology
- food chain
- bioaccumulation
- uranium
- polonium
- radiolead
- lichen
- reindeer
- radionuclide exposure
1. Introduction
Following the atmospheric nuclear tests in the 1950s and early 1960s, several radioecological research projects focusing on the (sub)arctic food-chain lichen-reindeer/caribou-man were initiated in Scandinavia and North America [1,2,3]. Lichen collects deposited radionuclides efficiently and is the main fodder for reindeer during the winter season. The enrichment of radionuclides in this food chain can lead to exceptionally high body burdens among the indigenous Sami and Inuit populations consuming large quantities of the meat and edible organs of reindeer and caribou. This concerns both artificial and natural radionuclides, even though the first research projects dealt with 137Cs and other fission product nuclides. However, it was observed that the natural radionuclides 210Pb and 210Po are enriched in this food chain as well.
210Pb is formed in the atmosphere from the radioactive noble gas 222Rn, emanating from the Earth’s crust. In total, 99% of the airborne 222Rn originates from land and only 1% from the sea [4], and consequently high 210Pb concentrations are found in continental air masses. Owing to the long half-life of 210Pb (t½ = 22.3 a), its removal from the atmosphere is governed by the wet and dry deposition processes affecting the aerosol particles carrying it rather than radioactive decay. 210Pb decays to 210Po (t½ = 138.4 d) via the short-lived beta emitter 210Bi (t½ = 5.013 d). Being an alpha emitter 210Po is highly radiotoxic.
From the radiation protection point of view, less important natural radionuclides are 238U, 226Ra, 40K, and Th isotopes. However, 238U is a long-lived (t½ = 4.5 × 109 a) alpha emitter whose chemical toxicity exceeds its radiological toxicity. Although isotope 238U is not considered to cause significant radiation exposure, it is still the mother nuclide of a whole uranium decay series, producing isotopes of astatine, bismuth, lead, polonium, protactinium, radium, radon, thallium, and thorium while decaying. 40K also has a very long half-life (t½ = 1.28 × 109 a). A human body has a 40K whole body content of a few kBqs. The alpha emitter 226Ra (t½ = 1600 a) is an important radionuclide considering ingested radiation doses. However, the population in Finland receives exposure to 226Ra mainly via uptake from drinking water, and there is hardly any published data about 226Ra in terrestrial environment and biota in Finland, except 226Ra concentration in cereals [5], animal-based food products [6], and timber [7]. Therefore, 226Ra is left outside the scope of this article. Thorium has several isotopes with widely different half-lives. The longest half-life belongs to isotope 232Th (t½ = 1.405 × 1010 a). Owing to the long half-life, the specific activity of 232Th is low and thus its potential threat against human health might be due to its chemical characteristics (heavy metal) rather than radiological hazards.
2. 210Pb and 210Po
According to Paatero et al. [9], the 210Pb deposition in northern Finland is about 20–40 Bq/m2/a (Figure 1). The deposition level is lower than in southern Finland, where the maximum deposition is about 80 Bq/m2/a. In the atmosphere the 210Po:210Pb, activity ratio is usually about 5–10%, so the amount of 210Po deposition in Finnish Lapland is about 2 Bq/m2/a [10]. However, the 210Po:210Pb activity ratio continues to increase after the deposition.
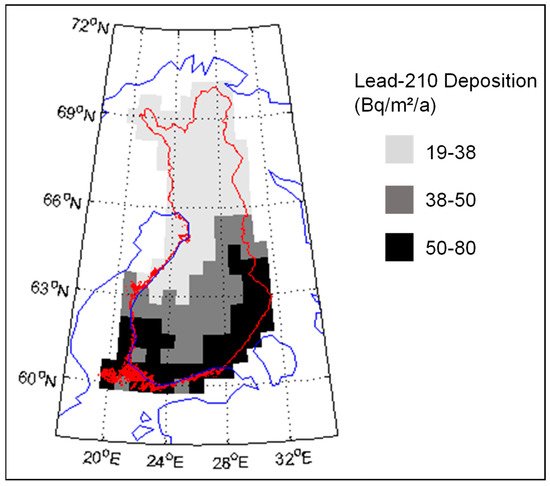
Figure 1. Deposition of 210Pb (Bq/m2/a) in Finland. Original data is published in [9].
210Po is enriched from soil to plants via root uptake [11,12] and in less extent via foliar uptake. Lichens behave differently than plants as 210Po transfers and enriches from air and surface soil to lichens mainly by surface (aerial) uptake [13]. 210Pb, a grandmother radionuclide of 210Po, has a different chemical and radiochemical behaviour than 210Po, although some similarities occur. Just like 210Po, 210Pb has also been found to accumulate efficiently to lichen, similarly with stable lead [14]. However, as will be discussed next and can be seen from the data in Table 1 and Table 2, there are clear differences between 210Po and 210Pb in their bioavailability and enrichment in the food chains.
Table 1. Concentrations of 210Pb in Finnish environmental and biological samples. d.w. = dry weight, w.w. = wet weight. Sampling year is in parentheses.
| Sample Type | A 210Pb (Bq/kg d.w.) | A 210Pb (Bq/kg w.w.) | Reference |
|---|---|---|---|
| Lichen (1961–1967) | 190–380 | [15] | |
| Lichen (1980) | 250 | [16] | |
| Lichen (2004) | 170 | [16] | |
| Tree leaves (1964–1965) | 11–42 | [15] | |
| Tree leaves (1989) | 20 | [16] | |
| Mushroom (2004) | 3 | [16] | |
| Mushrooms (2007) | 1.6–16 | [17] | |
| Berries (2004) | 2 | [16] | |
| Berries (2006–2007) | 0.09–0.45 | [17] | |
| Reindeer blood (1965–1967) | 1.5–3 | [15] | |
| Reindeer meat (1965–1967) | 0.12–0.30 | [15] | |
| Reindeer muscle (1984–2004) | 1.2–4.8 | [16] | |
| Reindeer bones (1964–1966) | 138–196 | [15] | |
| Reindeer bones (1988–2004) | 38–210 | [16] | |
| Reindeer kidney (1988–1989) | 62–85 | [16] | |
| Reindeer liver (1964–1967) | 10–56 | [15] | |
| Reindeer liver (2004) | 150 | [16] | |
| Human blood, reindeer herder (1966) | 0.23–0.27 | [15] | |
| Human blood, southern Finland (1966–1967) | 0.1 | [15] | |
| Placenta, Lapland (1966) | 0.02–0.12 | [15] | |
| Placenta, southern Finland (1966) | 0.01–0.05 | [15] | |
| Human kidney, southern Finland (~1966) | 0.12–0.24 | [15] | |
| Human teeth, Lapland (1966–1967) | 2.4–11 | [15] | |
| Human teeth, southern Finland (1966–1967) | 1.4–2.6 | [15] | |
| Human shinbone/tibia, southern Finland (~1966) | 1.0–1.5 | [15] | |
| Human ribs, Lapland (1977) | 0.85 and 1.5 | [18] | |
| Human liver, Lapland (1977–1979) | 0.13–1.0 (average 0.48) | [18] | |
| Human liver, southern Finland (~1966) | 0.08–0.11 | [15] | |
| Human liver, southern Finland (1976–1979) | 0.27 (average) | [18] |
Table 2. Concentration of 210Po in Finnish environmental and biological samples. Sampling year is in parentheses.
| Sample Type | A 210Po (Bq/kg d.w.) | A 210Po (Bq/kg w.w.) | Reference |
|---|---|---|---|
| Lichen (1961–1967) | 170–350 | [15] | |
| Tree leaves (1964–1965) | 3–12 | [15] | |
| Tree leaves (1989) | 19 | [16] | |
| Mushroom (2004) | 140 | [16] | |
| Mushrooms (2007) | 9–2190 | [17] | |
| Berries (2004) | 2.2 | [16] | |
| Berries (2006–2007) | 0.3–1.0 | [17] | |
| Reindeer blood (1965–1967) | 4–18 | [15] | |
| Reindeer meat (1964–1967) | 3–12 | [15] | |
| Reindeer muscle (1984–2004) | 23–66 | [16] | |
| Reindeer bones (1964–1966) | 73–78 | [15] | |
| Reindeer bones (1988–2004) | 12–71 | [16] | |
| Reindeer kidney (1988–1989) | 120–160 | [16] | |
| Reindeer liver (1964–1967) | 38–174 | [15] | |
| Reindeer liver (2004) | 470 | [16] | |
| Human blood, reindeer herder (1966) | 0.24–0.57 | [15] | |
| Human blood, southern Finland (1966–1967) | 0.03 | [15] | |
| Placenta, Lapland (1966) | 0.63–2.8 | [15] | |
| Placenta, southern Finland (1966) | 0.05–0.16 | [15] | |
| Human kidney, southern Finland (~1966) | 0.34–0.95 | [15] | |
| Human teeth, Lapland (1966–1967) | 2.3–10 | [15] | |
| Human teeth, southern Finland (1966–1967) | 1.4–2.5 | [15] | |
| Human shinbone/tibia, southern Finland (~1966) | 0.95–1.3 | [15] | |
| Human ribs, Lapland (1977) | 0.8 and 1.0 | [18] | |
| Human liver, Lapland (1977–1979) | 0.4–8.0 (average 3.2) | [18] | |
| Human liver, southern Finland (~1966) | 0.34–0.83 | [15] | |
| Human liver, southern Finland (1976–1979) | 0.57 (average) | [18] |
Lichen is the main fodder of reindeer during the winter. In the 1960s, the 210Pb activity concentration in lichen (Cladonia alpestris) varied between 210 and 380 Bq/kg dry weight (d.w.) in Finnish Lapland and between 190 and 300 Bq/kg d.w. in southern Finland [15]. The 210Po:210Pb activity ratio in lichen varied from 0.78 to 0.98. The activity concentrations of 210Pb and 210Po were about a factor of 50 higher in lichen compared to those in other plants that reindeer eat during the summer season, such as birch (Betula verrucosa/Betula pendula) leaves. Persson [19] found similar 210Pb activity concentration levels in lichen in Sweden. According to his studies, the effective half-life (physical + biological) of 210Pb in lichen is 7 ± 2 years. As 210Pb and 210Po are natural radionuclides, their amount in the environment is very stable in the long-time scale. Due to this, the 210Pb content in lichen has not changed during the several decades of monitoring. The activity concentration of 210Pb in ground lichen (Cladonia sp.) was 250 Bq/kg d.w. in western Lapland in 1980 and 170 Bq/kg d.w. in eastern Lapland in 2004 [16].
Reindeer consume mushrooms in late summer and autumn. The 210Pb content in brown-yellow boletus (Suillus luteus) is rather low, only a few Bq/kg d.w. However, they contain 100–140 Bq/kg d.w. of 210Po, which significantly increases the intake of this nuclide into reindeer [16]. The content of 210Pb and 210Po varies two orders of magnitude from one species to another in mushrooms sampled in northern Finland (Figure 2a,b) [17]. The activity concentration of 210Pb ranged from 1.55 (Leccinum vulpinum) to 16.2 (Cortinarius armillatus) Bq/kg d.w. For 210Po, the corresponding range was from 8.98 (Hygrophorus camarophyllus) to 2192 (Leccinum vulpinum) Bq/kg d.w., respectively. Additionally, the type of forest and forest soil affects the content of 210Pb and 210Po in mushrooms. For example, the concentration level of radionuclides in the organic-rich litter layer affects the radionuclide content of mushrooms, but a clear connection between soil type and concentrations of 210Pb and 210Po in mushrooms has not been established yet. Furthermore, mushroom species are suggested to be the most important factor in radionuclide accumulation [17].
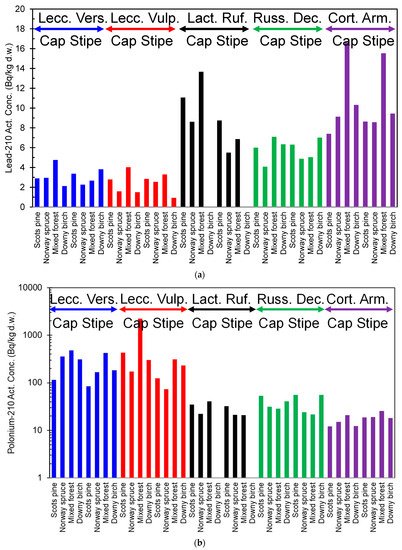
Figure 2. (a). 210Pb activity content of different mushroom species (blue = Leccinum Versipelle, red = Leccinum Vulpinum, black = Lactarius Rufus, green = Russula Decolorans, and purple = Cortinarius Armillatus) in northern Finland, from the data in [17]. (b). 210Po activity content of different mushroom species (blue = Leccinum Versipelle, red = Leccinum Vulpinum, black = Lactarius Rufus, green = Russula Decolorans, and purple = Cortinarius Armillatus) in northern Finland, from the data in [17].
The activity concentration of 210Pb and 210Po in different parts of blueberry and lingonberry in northern Finland is depicted in Figure 3 [17]. The activity concentration of 210Po is higher than that of 210Pb in stems, berries, and leaves of both berry species, indicating a higher bioavailability of polonium compared to lead. The berries have much smaller activity content of both radionuclides compared to other parts of the plants. Plants are known to have a selective ability to metal uptake, capacity to store toxic metals in inert parts (e.g., tree stems) and seedlings, and prevention mechanism for blocking accumulation of toxic metals in their reproductive organs, e.g., seeds and berries [20,21,22,23,24].
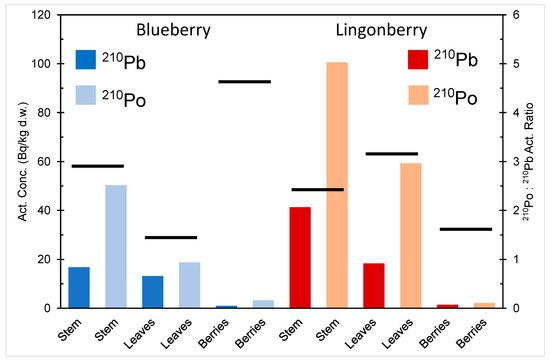
Figure 3. 210Pb and 210Po activity content in different parts of blueberry (V. myrtillus) and lingonberry (V. Vitis-idaea) in northern Finland, drawn from the data in [17]. The black lines denote the 210Po:210Pb activity ratios: a vertical scale on the right.
The seasonal variation of reindeer’s feeding habits are also reflected in the content of 210Pb and 210Po in various reindeer tissues (Figure 4). The activity concentrations are in their minimum in summer when reindeers have consumed vascular plants, birch leaves, etc. (Figure 5 and Figure 6). The activity concentrations are higher in autumn and winter after the consumption of first mushroom and, later, lichen [15].
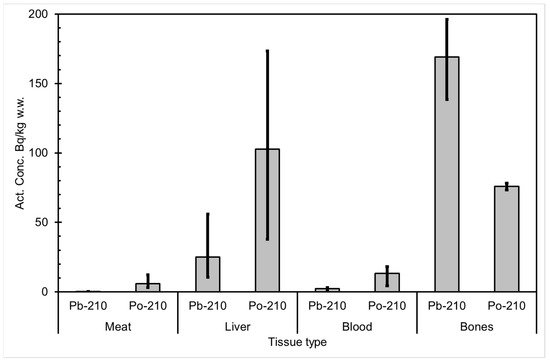
Figure 4. Average 210Pb and 210Po activity concentrations (Bq/kg wet weight) in reindeer tissues, compiled from data in [15]. The black vertical lines indicate the range of individual values.
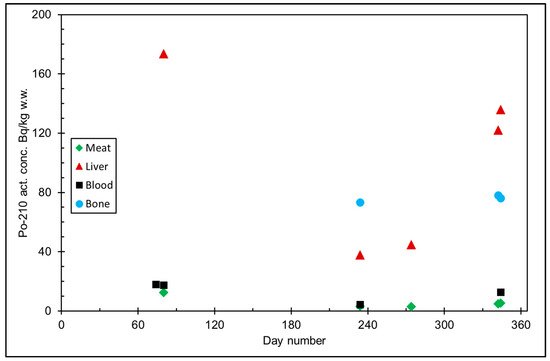
Figure 5. 210Po activity concentration (Bq/kg w.w. = wet weight) in reindeer tissues, from the data in [15]. Day number refers to Julian day number.
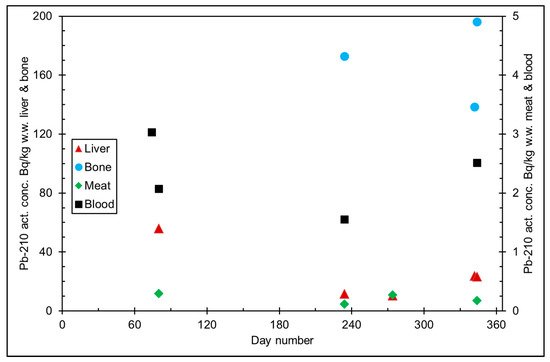
Figure 6. 210Pb activity concentration (Bq/kg w.w. = wet weight) in reindeer tissues, from the data in [15]. Day number refers to Julian day number.
According to results by Solatie et al. [16], 210Pb mainly enriches reindeer bones after entering to reindeer body. There was a 10-fold concentration of 210Pb in the reindeer bones compared to muscles. In the same study, the concentration of 210Po was two times higher in bones than in muscles, at most. Therefore, relative enrichment of 210Po to bones was not as high as of 210Pb in investigated samples. Alongside bones, both 210Pb and especially 210Po enrich the liver and kidney in reindeer. The same preference for enrichment of 210Pb was also observed with aribous in Canada [13]. In past centuries when reindeer was the foundation of diet among reindeer herders, consumption of inner organs and bone marrow of reindeer was also considerable; therefore, it has had a significant effect on intake of both 210Pb and 210Po, increasing the internal radiation dose in the Lapland region.
Enrichment of 210Pb and 210Po from diet to humans is roughly similar to reindeer, since both radionuclides accumulate to human bone, liver, and kidney. Next, some differences between the two regions (Lapland and southern Finland) with different diets in the past, in the concentrations of 210Pb and 210Po in human organs, are discussed. A related review about possible increased cancer risk among Sami people due to anthropogenic radionuclides from nuclear weapons tests, diet, and life habits has been recently published by Soininen and Mussalo-Rauhamaa [25].
Kauranen and Miettinen [15] estimated that the intake of 210Po via ingestion within the reindeer herding population, 2.6 Bq/d, was 20 times higher than in the case of the general population, having an average western diet of 0.12 Bq/d. The corresponding intake of 210Pb was three times higher, 0.32 Bq/d, within the reindeer herding population, than in the average western diet of 0.12 Bq/d. The high intake of 210Po was mainly due to 210Po in reindeer meat and liver. In recent decades, the difference in diet habits between reindeer herders vs. the average population has probably decreased but not entirely disappeared. The high 210Po content in diets is also reflected in the body content of 210Po within the reindeer herding population. The 210Po activity concentration in the blood of reindeer herders was almost 20 times higher than within the population of Helsinki. The difference in the case of 210Pb was almost threefold. Correspondingly, the activity concentration of 210Po in human placenta in Inari and Utsjoki was over 12 times higher than within the population of Helsinki. In the case of teeth, the difference was only about a factor of two; the same as with 210Pb. It is clear that 210Po is not accumulated into teeth but is formed in situ from the decay of 210Pb.
In a later study by Mussalo-Rauhamaa et al. [18], a set of tissue samples of five autopsy cases from Inari and Utsjoki region, deceased between 1977 and 1979, were analyzed. It was found that the 210Pb activity concentration in human liver ranged from 0.13 to 1.0 Bq/kg wet weight (w.w.) with an average value of 0.48 Bq/kg w.w. In autopsy cases from southern Finland, an average 210Pb activity concentration of 0.27 Bq/kg w.w. was obtained. 210Po activity concentration in human liver in Lapland ranged from 0.40 to 8.0 Bq/kg w.w., with an average of 3.2 Bq/kg w.w. In autopsy cases from southern Finland, an average 210Po activity concentration of 0.57 Bq/kg w.w. was obtained. Thus, the activity concentration was two times higher in the residents of Lapland compared to those in southern Finland in the case of 210Pb. In the case of 210Po, the average activity concentration was over five times higher in the residents of Lapland compared to those in southern Finland. In a recent study, Muikku et al. [26] reported that the 210Po content in urine was three times higher in reindeer herders than the control group representing the average population. However, in the case of 210Pb, there was no difference.
The work of Holm and Persson [27] indicates clearly how 210Po dominates the total alpha activity of lichen (Table 3). The activity concentration of 210Po in 1972 was 50 times higher than the infamous plutonium isotopes from the atmospheric nuclear tests. In addition, the deposition of 210Pb has recurred every year after the ice age and will recur in the future too, contrary to plutonium deposition, which was in a longer timescale and was practically a single event. Furthermore, the half-lives of artificial radionuclides 238Pu (t½ = 87.7 a), 244Cm (t½= 18.1 a), and 242Cm (t½ = 163 d) are so short that they have decayed more or less significantly since their introduction to the environment.
Table 3. Alpha-emitting radionuclides in lichen in Sweden in 1972 (original data from Holm and Persson [27]) and relative activities compared to 238U.
| Radionuclide | Activity Concentration (Bq/kg d.w.) | Relative Activity (Act. Conc. 238U = 1) |
|---|---|---|
| 210Po | 260 | 1300 |
| 234U | 0.2 | 1 |
| 235U | 0.007 | 0.035 |
| 238U | 0.2 | 1 |
| 228Th | 0.13 | 0.65 |
| 230Th | 0.06 | 0.3 |
| 232Th | 0.06 | 0.3 |
| 238Pu | 0.23 | 1.15 |
| 239+240Pu | 5.2 | 26 |
| 241Am | 1.1 | 5.5 |
| 237Np | 0.01 | 0.05 |
| 242Cm | 0.0002 | 0.001 |
| 244Cm | 0.002 | 0.01 |
After 1972, there have been several other nuclear events releasing artificial radionuclides to the environment, most importantly the nuclear reactor accidents of Chernobyl in 1986 and Fukushima in 2011. This kind of accidental release of radionuclides creates local and regional elevated radiation exposure. The radionuclide(s) in question, as well as the released activity level, environmental conditions, and countermeasures after the accident, etc., determine how high fractions of the received radiation dose are from natural radionuclides and from accidentally released (often artificial) radionuclides. The early situation after a nuclear accident, where artificial radionuclides often dominate the natural radionuclides as an exposure source, may change during time, when shorter-lived artificial radionuclides have been decaying and both spontaneous environmental processes, as well as remediation actions, have occurred.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph182010577
This entry is offline, you can click here to edit this entry!
