Due to the limited reaction rate of the oxygen reduction reaction (ORR), it is considered as a limiting factor in the performance of fuel cells and metal-air batteries. Platinum is considered the benchmark catalyst for ORR; however, the scarcity of platinum, its high price, the drift phenomenon, its insufficient durability, and its susceptibility to gas poisoning are the reasons for the constant search for new ORR catalysts. Carbon-based catalysts show exceptional promise in this respect considering economic profitability and activity, and, in addition, they have favorable conductivity and often a large specific surface area.
1. Introduction
In recent years, the awareness of the human influence on climate change has increased. More and more attention is being paid to factors leading to global warming, especially CO
2 emissions. As fossil fuel usage leads to CO
2 release into the atmosphere [
1], in addition to their limited availability [
2], research groups worldwide are dedicated to finding alternatives to the carbon cycle. Fuel cells are of extraordinary importance in this respect, as they can participate in the hydrogen cycle. Fuel cells, as devices that convert oxygen and chemical energy into electricity with a high efficiency, are considered environmentally friendly and can be used for small, portable electronic devices and military and space devices [
3,
4,
5]. Metal-air batteries represent cost-effective devices that require an air atmosphere [
6], and the most promising are Zn-air batteries, with a high theoretical energy density, solid rechargeability, and flat discharge voltage [
7,
8]. During discharge, the reduction of oxygen at the cathode occurs while the metal oxidizes and releases electrons which pass through the external circuit in metal-air batteries. In the fuel cells, the H
2 dissociation occurs at the anode, while at the cathode, O
2 reduction occurs, transforming chemical energy into electricity [
9]; thus, oxygen reduction reaction (ORR) is the most important cathode reaction for both types of devices [
10,
11,
12].
The ORR catalyst is the main descriptor for the performance of these devices, with conventional platinum still regarded as the best ORR catalyst in both acid and alkaline electrolytes. However, Pt has a high price, a susceptibility to time-dependent drift, and serious anode crossover, and it is easily poisoned by CO (an intermediate product of electro-oxidizing alcohol fuels), which limits its usage. The limited availability of Pt is another important fact and constrains the possibility of using devices whose operation is based on ORR [
13,
14]. Therefore, platinum has to be replaced with appropriate alternatives that have a similar catalytic activity and can be produced massively and cost-effectively from Earth’s abundant resources [
15]. Electrocatalysts should have high electrical conductivities, large specific surface areas, and electroactive properties [
11,
16]. Conductive polymers (CPs) and carbon materials (grapheme, carbon nanotubes (CNTs), amorphous carbon, and carbon nanofibers) can fulfil these requirements, and they have been widely explored for oxygen reduction in fuel cells, among other applications [
13,
17].
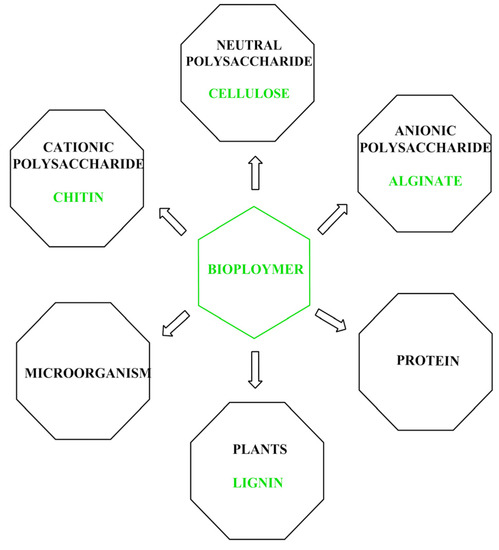
Biopolymers, substances consisting of numerous repeating monomer units, are present in natural sources. In
Table 1, the principal advantages and disadvantages of natural biopolymers are presented [
54,
55]. These are promising candidates for different spheres of medicine and industry due to their biocompatibility and biodegradability. The biopolymers are used as edible films, emulsions, packaging materials in the food industry, drug transport materials, medical implants, tissue scaffolds, dressing materials in pharmaceutical industries, etc. [
55].
Table 1. The positive and negative aspects of natural biopolymers.
The following sections will discuss the use of some biopolymers-based materials as promising candidates for ORR catalysis, whose performance was most often tested in alkaline conditions. Those that are applied most often are alginate, cellulose, chitin, and lignin due to their broad availability and low price [
56,
57,
58,
59,
60,
61].
Figure 1 represents the biopolymer categorization [
55] with respect to the biopolymers considered herein.
Figure 1. A pictorial depiction of several natural, renewable biopolymers categorized according to their source [
55].
Major challenges during the synthesis of ORR catalysts include the efforts to extract biopolymers from the biomass, along with methods for cleaning and decomposition, simplifying the conversion processes with the aim of ensuring the low cost of synthesis and production on a bigger scale. Knowledge of active sites and the relationship between the structure and mass transfer can facilitate the selection of materials and the methodology for ORR synthesis. Additionally, it is necessary to intensively transfer the half-cells tests of synthesized ORR catalysts to corresponding devices, such as fuel cells and metal-air batteries [
13].
2. Alginate as a Source of Carbon Material
Biopolymers, as renewable and non-toxic precursors, are important for the synthesis of carbon materials. Abundant sea resources represent one of the most important sources, considering that the ocean occupies three-quarters of the Earth’s surface [
63].
The wide alginates availability, with the simplicity of their extraction and synthesis via “green” processes, makes them exceptionally favorable materials, both commercially and environmentally. The field of their applications and significance is expanding, and one of the main challenges is their use as precursors of carbon materials production on a large scale.
Brown algae are a valuable source of alginate, a natural polymer composed of β-D-mannuronate and α-L guluronate. Due to the structure, as alginate consists of a large number of carboxyl and hydroxyl groups in the polymer chain, it is possible to obtain porous carbon material after alginate carbonization [
64,
65]. However, the different sequence and structure that vary depending on the source may be a disadvantage when this biopolymer is used [
66]. Alginate interacts with metal ions and can chelate divalent and trivalent ions such as Ca
2+, Co
2+, Ni
2+ Zn
2+, and Fe
3+, consequently forming an “egg-box” structure that can be used for the synthesis of metal-doped/free three-dimensional carbon nanomaterials with multimodal pores. This represents one of the main advantages of using the alginate as a precursor considering that the 1D structure has a very pronounced limited mass transfer of electrolyte ions. Multimodal pores formation was reported as a result of the material acid rinsing after thermal treatment in an inert gas atmosphere [
67,
68]. Large pores corresponded to the elimination of metal chelated into alginate, while smaller pores were the result of the release of H
2O and CO
2 during the thermal treatment.
A comparative table with the indicated method for obtaining the type of carbon material and the characteristics of the ORR catalysts derived from alginate is given in Table 2.
Table 2. ORR catalysts derived from alginate; characteristics, methods for obtention, and type of carbon materials.
3. Other Widely Used Biopolymers
Cellulose, lignin, and chitin are widely distributed polymer materials that are increasingly used as sustainable precursors for the synthesis of carbonaceous materials since they are non-toxic, biodegradable, and biocompatible [
103,
104].
3.1. Cellulose
Cellulose is a homopolymer consisting of glucopyranose [
105]; therefore, to obtain materials with good ORR electrocatalytic activity, doping or co-doping with heteroatoms is desirable [
51]. The chains of cellulose are connected in parallel, and the crystalline structure, via hydrogen and van der Waals interactions, forming microfibrils [
51]. The degree of polymerization varies according to its source [
106] Besides many advantages, such as the large amounts, biodegradability, low cost, etc., they also have some disadvantages; the low moisture resistance may be the most important [
107].
Kim et al. used cellulose as the biomaterial for the high-value-added N-doped hierarchical porous carbon (NHPC) [
108]. They developed a simple, inexpensive, and efficient process in which they mixed cotton cellulose with magnesium nitrate hexahydrate or magnesium acetate tetrahydrate and urea in different proportions. As previously reported methods included high-cost materials and low outputs, this method presents a great improvement. Upon drying, the mixture was pre-pyrolyzed at 500 °C in an Ar atmosphere. Afterwards, the obtained material was carbonized at different temperatures. The authors explained in detail the influence of the preparation parameters and contributed to the improvement of the methodology of making carbon-based ORR catalysts. Pyrolysis and carbonization were separated in order to characterize the material after pyrolysis and to avoid the pollution of high-temperature furnaces due to a large amount of gas emission. The treatment with an acidic solution was applied after the carbonization with the aim of obtaining a final material without metal ions.
The novelty of the proposed method was that the authors used accelerated pyrolysis, where the exothermic reactions were caused by the application of nitrates, which encouraged pyrolysis at low temperatures and the rapid exfoliation of cellulose fibers. In addition, urea was used as an additional source of nitrogen but also as a reactant that supports the total exfoliation of cellulose when applied in the optimal content. Consequently, the sample synthesized with magnesium nitrate and urea had a highly 3D porous structure with macropores, mesopores, and abundant micropores and a specific surface area of 1173 m
2 g
−1. Graphitic and pyridinic N, i.e., the N-C parts of the catalysts, were considered as active sites, and the high specific surface area combined with the hierarchical open pore structure contributed to the increase in the exposed active sites, thus enabling efficient mass transport and fast ion transport. According to the electrochemical measurements, including CV and LSV, the onset potential was 0.94 V, the half-wave potential was 0.83 V, and the estimated number of transferred electrons was in the range of 3.5–4 for the sample carbonized at a temperature of 1000 °C. As the temperature of carbonization influences the doping amount and doping species of N, as the graphitization degree of the carbon, according to the stated results, the optimal temperature was 1000 °C. The durability of the optimal material was better compared to that of the Pt/C catalyst, as the current obtained by the chronoamperometric measurements was 88% after 10 h in KOH, while for the Pt/C, it was 83% at 0.6 V vs. RHE in the O
2-saturated KOH solution at a rotating speed of 1600 rpm. The material was methanol-tolerant, as the current had no significant changes after the addition of 2% methanol, while for the Pt/C, the current decreased [
108].
3.2. Chitin
Chitin is, besides cellulose, the most widespread biopolymer [
109,
110]. It has a high content of nitrogen. Before using from the biomass, the chitin has to be extracted. This process includes mechanical grinding, chemical demineralization, and deprotonation, techniques that may be time-demanding. In addition, the direct carbonization of chitin could not provide a defined morphology and porosity of the newly obtained material [
111]. Chitosan, the deacetylated form of chitin with a different degree of deacetylation, may also be used for different purposes [
110,
112]. As chitosan contains about 7% of N, it presents a valuable resource of material containing carbon and nitrogen from amine and acetamide functional groups [
57,
103,
113,
114], which, upon thermal treatment, may exclude an additional step of N-doping in the synthesis process of functionalized carbons.
The specific surface area and the type of functional groups [
115], both dependent on the carbonization temperature, are the main factors favoring an increase in the catalytic activity for N-doped porous carbons obtained from chitin and chitosan. The urea treatment of these materials had a great benefit for their ORR performance, since it further increased the specific surface area and enhanced the N-doping [
116].
Wang et al. designed cobalt- and nitrogen-co-doped carbon material (CoNC), using a mixture of chitin and cobalt as a precursor, through a one-step pyrolysis process at 800 °C [
117]. The obtained CoNC showed a specific area of 165 m
2 g
−1, and it was reported as a good material for ORR electrocatalysts in alkaline media for Al-air batteries. The onset potential was 0.86 V vs. RHE, and the high-limiting current density of 4.91 mA cm
−2 was comparable to those obtained for Pt/C 0.9 V and 5.67 mA cm
−2. The electron-transfer number was 3.73. The long-time durability was examined at 900 rpm in 0.1 M KOH saturated with O
2, and after 15,000 s, the current density was 94.82%.
3.3. Lignin
Lignin is the by-product obtained in the process of pulping. It is the constituent of grass, trees, and plants [
118].
Lignin presents an amorphous aromatic polymer [
58] which consists of p-hydrophenyl (H), syringyl (S), and guaiacyl (G) components, which form a three-dimensional structure. As lignin has a high content of benzene rings and phenolic functional groups, it is widely used as a precursor for the synthesis of carbon material (carbon spheres, nanofibers, nanosheets, 3D-porous carbon, carbon composites, etc.). The availability, low cost, the unique designability and controllability of the lignin structure are certainly its great advantages for its usage in different spheres [
58]. The disadvantages of lignin include the fact that its usage may be a complicated procedure that disables the large-scale production. Accordingly, the development of an environmentally friendly process for the production of carbon materials from lignin is mandatory, with special attention given to the fact that the relationship between the structure of carbonized lignin and its catalytic activity is not absolutely understood yet.
The reported carbon materials derived from lignin include nitrogen-doped and nitrogen/sulfur- and nitrogen-phosphorus-co-doped catalysts [
119,
120,
121]. Li et al. [
122] used lignosulfonate and synthesized a robust multifunctional carbon catalyst for ORR, OER, and hydrogen evolution reaction (HER), which contained iron, nitrogen, phosphorus, and sulfur. Although different methods for carbon-doping were reported, the catalytic activity for the ORR, OER, and HER of such materials was not satisfactory. The improvement was achieved by the synthesis of the catalyst with a larger specific surface area, where active sites were exposed to the surface. The highly porous structure improved the rate of mass/electron transfer, the doping with heteroatoms upgraded the electroconductibility and charge transfer, and the FeN
x and FeP
x species had an impact on the prevention of the agglomeration of active sites and thus provided great durability. Finally, the obtained catalyst had the intrinsic activity of each active site and, consequently, great activity toward these three different reactions. The material was synthesized during the process that consisted of the preparation of lignin-Fe, and then the obtained substance was mixed with hypophosphite (inorganic molten salt template), annealed at 500 °C, and carbonized at 800 °C. After treatment with HCl solution, the material was again annealed at 800 °C and tested for catalytic activity [
122]. The obtained catalyst had a specific surface area of 782 m
2 g
−1, which is larger than that of some reported catalysts [
121]. The half-wave potential was 0.9 V, and the electron transfer number was 3.9. After injecting 3 M methanol, the current density had a negligible change, indicating a robust tolerance to methanol [
122]. After 86,400 s, the current changed by 9.1%, while for the Pt/C, the change was 17.1%. Shen et al. [
121] used lignin with melamine to form lignin carbon nanosheets of a surface area of 1208 m
2 g
−1 co-doped with nitrogen and sulfur. The material may be used in an acid medium as well as in alkaline due to its better performances compared to Pt/C (a more positive half-wave potential and (nearly) current density and a high graphitic N ratio including four electron mechanisms for ORR).
This entry is adapted from the peer-reviewed paper 10.3390/catal13010080