Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Among primary liver cancers, hepatocellular carcinoma (HCC) is the most common. Surgical resection and liver transplantation both represent potentially curative treatments not only in the case of the first occurrence, but also in those cases of disease recurrence if a proper selection of patients is performed ahead. Incidentally, the type and the time of relapse carry important weight on patient prognosis and overall survival. For these cases, proper management has still not been exactly defined.
- hepatocellular carcinoma
- recurrent hepatocellular carcinoma
- salvage liver transplant
- redo hepatectomy
- liver resection
- surgical management
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumour and the fifth leading cause of cancer-related death worldwide [1]. Most of the time, HCC develops on a liver cirrhosis substrate [2], with hepatitis B virus representing the most common risk factor in about 50% of cases [3]. The risk attributable to hepatitis C virus infection has been decreasing due to new antiviral drugs [3][4], while metabolic syndrome leading to fatty-liver disease and non-alcoholic steatohepatitis is becoming a remarkable growing cause, particularly in Western countries [5].
Primary liver resection (PLR) and primary liver transplant (PLT) are considered potentially curative options [6][7]. However, recurrent HCC (rHCC) after surgical treatment represents a major challenge with a median 5-year survival after recurrence (SAR) of about 35% [8].
To date, numerous articles have been published focusing on recurrence risk factors, showing many different independent variables [9][10], such as those related to tumour pathology (i.e., micro- or macrovascular invasion, presence of satellitosis, differentiation grade) and those related to the underlying liver substrates (i.e., grade of fibrosis, hepatitis viral infections) [11]. Furthermore, all these factors are inherently related to the most recent clonal origin interpretation [12], which indicates two main time frames for the development of rHCC: early recurrence, meaning any recurrence within 2 years of resection, which has been associated with tumour factors; and late recurrence, meaning any recurrence after 2 years of resection, which has been associated more with underlying cirrhosis [11]. However, other authors reported different time frames behind the development of rHCC [13][14][15], indicating that more studies on rHCC development should be performed.
While great attention has usually been posted on treatment algorithms for the first HCC [16][17], standardized indications for rHCC are lacking. Thus far, only one proposal from an International Eastern and Western consensus has been published [18]. At the same time, new studies have been recently published on the role of redo surgery in cases of rHCC [19]. These, together with those studies on salvage liver transplantation (SLT) [20][21], appear to be good to take stock of this argument.
2. Pattern of Recurrence and Prognostic Significance
After a first curative treatment, HCC has a 5-year recurrence rate of up to 70% [22][23]. The recurrence is related to several different factors. Other than the eventually underlying liver hepatopathy, there are primary tumour characteristics strictly related to aggressiveness and risk of relapse, hesitating in poor survival [24][25]. In terms of pathological appearance, several HCC subtypes have been defined with associated clinical and genetic features [26][27]. The 5th WHO classification defines eight HCC subtypes based on histopathological morphology: steatohepatitic, clear cell, macrotrabecular-massive, scirrhous, chromophobe, fibrolamellar carcinoma, neutrophil-rich, and lymphocyte-rich [27]. On the other hand, six different subclasses (G1–G6) have been distinguished based on gene expression profiles [28]. Combining these characteristics, a particular risk profile could be delineated, as largely detailed, in relation to tumour aggressiveness [24][28]. Among the risk factors, vascular invasiveness (as in the macrotrabecular subtype), TP53 enrichment (G1–G3 subclasses), sarcomatous changes (as in the scirrhous-sarcomatoid subtype) were associated with poor prognosis. On the other hand, chromosomal stability (G4–G6 subtypes) and lymphocytic over neutrophil infiltration (as steatohepatitic) were associated with good prognosis [24][26].
Moreover, recurrence may be characterised by different patterns of relapse considering either the time to recurrence (early versus late) or the type of treatment received (PLR versus PLT). HCC may relapse within the liver (intrahepatic recurrence, IHR) or in extrahepatic sites (EHR).
IHR is more often the major challenge after a PLR; notably, it is associated with the number and size of HCC nodules as well as with the presence of micro- and macro-vascular invasion [29]. Conversely, EHR mainly consists of pulmonary nodules that, while not being as frequent, are associated with a poorer prognosis than IHR [30]. The latter type of recurrence is more associated with PLT rather than with PLR, particularly when PLT is performed beyond the Milan criteria [31] or in the presence of macrovascular-invasive HCC. HR and EHR may coexist.
In the last decade, increasing attention has been drawn to IHR patterns [32][33][34], as two different events have been described with distinct prognostic significances [14][35][36]. The first is the event of intrahepatic metastasis (IM) while the second is the event of multicentric occurrence (MO). Sakon et al. [37][38] further categorised IM events as follows: (1) local IM (Figure 1A), where the rHCC develops along the tumour blood flow (TBF) or venous drainage, and (2) systemic IM (Figure 1B), which considers the recurrence caused by circulating tumour cells (CTCs) in the rehoming of the remnant liver. Notably, this IM categorisation was clearly demonstrated in a large European series, where local IM was related to the presence of positive surgical margins after a first resection, while intra-hepatic distant relapse (systemic IM) was related to daughter nodules and microvascular invasion [39].
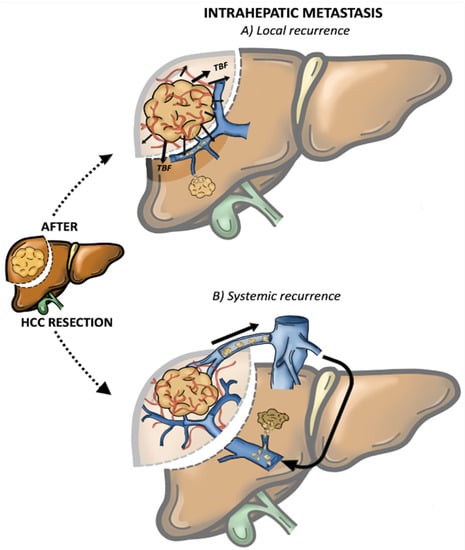
Figure 1. Intrahepatic metastasis (IM). (A) Local recurrence: the rHCC develops along the tumour blood flow (TBF) (B) Systemic recurrence: caused by circulating tumour cells (CTCs) in the rehoming of the remnant liver.
In contrast, MO appears to be more likely associated with de novo tumour formation due to its tardive recurrence [40]. In addition, MO usually consists of multiple nodular disease with well-differentiated carcinoma surrounding a less-differentiated mass (known as “nodule in nodule” form) and rare vascular involvement (Figure 2) [11][41][42].
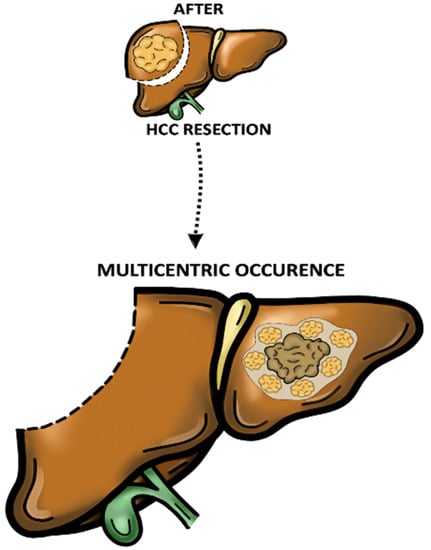
Figure 2. Multicentric occurrence (MO). Multiple nodular disease with well-differentiated carcinoma surrounding a less-differentiated mass (known as “nodule in nodule” form).
Consistently, in terms of relapse timing, early recurrence has been associated with the IM event, while late recurrence is associated with the MO event [11][13][15]. As is already known, in 2003, Imamura et al. [11] identified two peaks of recurrence after PLR: the earlier at 1 year (essentially following an IM mechanism) and the later at 4 years (categorised as MO aetiopathogenesis). Furthermore, three prognostic factors were associated with the early recurrence: non-anatomical resection, microscopic vascular invasion, and elevated serum alpha-fetoprotein (AFP) level > 32 ng/mL. On the other hand, a higher grade of hepatitis activity and well-differentiated multiple tumours were identified as factors related to the late recurrence [11]. These data were further confirmed by other groups [43][44], and a recent meta-analysis reported how the MO group had higher overall survival (OS) and disease-free survival (DFS) than the IM group [32].
Along with the aforementioned pathological features and recurrence, enabling the discrimination between IM and MO, great efforts have been spent on identifying molecular changes, such as clonal origin and genetic alterations, to better differentiate these two patterns [12][45]. In recent years, cutting-edge techniques such as next-generation sequencing, allowed for intertumoural genetic heterogeneity to be better defined, demonstrating that IM shared analogous molecular changes with the primary tumour, while MO displayed different genetic mutations [46]. In this scenario, Furuta et al. [47] proposed that a common mutation rate of less than 5% in HCC nodules indicated the MO pattern, while the IM was characterised by more than 5% of shared mutations. These results highlighted that the association of clinical parameters with tumour molecular analysis could improve the accuracy in the diagnosis and management of IM/MO.
Of note, no guidelines currently include histopathological, genetic and IM/MO patterns as part of the treatment algorithms for rHCC. This lack of guidelines highlights the need for future efforts in individualising postoperative surveillance and postoperative therapies.
3. Adjuvant Postoperative Treatments
Some studies have been conducted to test the role of adjuvant postoperative systemic treatments after PLR or PLT: as HCC is a chemo-resistant tumour, cytotoxic therapies failed to provide any survival benefit and have been abandoned [48]. Similarly, the multikinase inhibitor sorafenib was tested after PLR or ablation in the STORM study, which was a randomised controlled trial, but no effective advantages were recorded [49]. The more recently introduced Lenvatinib has not yet been tested in the adjuvant setting after PLR, with the only study reporting no benefits in terms of rHCC after PLT [50].
A different story might be associated with immune checkpoints inhibitors (ICIs) which are emerging as a treatment option for HCC. There might be a strong rationale for the application of ICIs to prevent rHCC after PLR or ablation since both treatments are known to increase immunogenicity [51]. PLR and ablation may cause the release of tumour-associated antigens and antigen-presenting cells that can activate the cytotoxic effects mediated by CD8+ T cells. This mechanism can be effective in those microscopic daughter nodules left behind a PLR. Such a scenario was reported by Duffy et al. [52], who showed the infiltration of CD8+ cells in untreated HCC nodules after ablation or trans-arterial therapies.
With this rationale, there are different ongoing studies on ICIs in the adjuvant setting after PLR, by using single agents, a combination of two or three agents, or a combination of immunotherapy and an anti-VEGF factor such as bevacizumab. However, some preliminary results are disappointing [53], mainly due to the evidence of the strong immunosuppressive tumour microenvironment of rHCC being associated with immunotherapy resistance [54][55].
Other than medical therapies, it deserves to be mentioned the emerging role of transarterial chemoembolization (TACE) in the adjuvant setting. Even with initial controversial results [56][57], TACE after curative PLR resulted in longer DFS and OS for selected patients with microvascular invasion, as reported from a recent meta-analysis [58]. Specifically, these results were confirmed when a subanalysis was performed in patients with microvascular invasion, multinodular disease and the largest tumour size greater than 5 cm, all risk factors associated with early recurrence [58].
At the moment, neither medical therapies nor interventional radiology techniques have been standardized or commonly recognized to prevent HCC recurrence after PLR. At the least, we must wait for the results of the ongoing clinical trials.
4. Surgical Treatments for rHCC
4.1. Redo Hepatectomy
In the case of HCC relapse, most physicians consider recurrence as failure of the curative treatment, hence addressing the patients to palliative care. However, increasing evidence underlines the chance to cure the recurrence, managing it as a first occurrence and resulting in the prolongation of OS [19]. Redo hepatectomy (RH) has been demonstrated as a valid option, as firstly described by Nagasue et al [59]. Due to the improvements in surgical techniques and perioperative care, Chan et al. [60] reported 90-day mortality to be a rare event, with a median rate of 0% (range 0–6%), indicating that the safety profile of RH is equivalent to that of PLR. The key for obtaining the most successful results is to select patients who may benefit more from a second surgical treatment. In this case, there are no clear guidelines, with few groups providing only general recommendations [61]. For this reason, most clinicians are led to restage the recurrence as a first occurrence [62] and several different factors should be considered in this management process [44], such as the number of recurrent nodules, nodule size, IHR or EHR, and gross vascular invasion. On the other hand, there are also factors related to the patient such as: age [18], the functional liver reserve estimable with different methods including the Child–Pugh–Turcotte score [63][64], the BILCHE score [65] or the indocyanine-green retention test [66], platelet count, the AFP-value at the time of recurrence [67] and the value of hepatic venous pressure gradient [68]. Balancing all of these factors, while only about 20% of all rHCC cases were surgically treatable, they were treated with minor hepatectomies in almost 99% of cases, which are interventions that are associated with a lower risk profile [69]. This was previously reported by Chan et al. [60], who finally proposed considering simplicity in the decision process leading to RH, with only two stronger predictors of poor survival outcomes: the presence of vascular involvement and the residual hepatic reserve.
The so-called “test of time”, which may have importance for the risk of developing a second recurrence after RH, is no less important. In this sense, some authors indicate how an early relapse should be regarded as an indirect expression that correlates with a worse outcome [11][13][19][70][71]. Nevertheless, DFS was the only factor found to be independently associated with survival in the systematic review as reported by Chan et al. [60].
Applying these aforementioned selection criteria, RH showed a median OS varying between 22.0 and 71.7 months and a median DFS ranging between 7.0 and 57.0 months as shown in Table 1[14][70][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92].
Table 1. Published papers on redo hepatectomy for recurrent hepatocellular carcinoma (after liver resection at first occurrence) in the last 20 years.
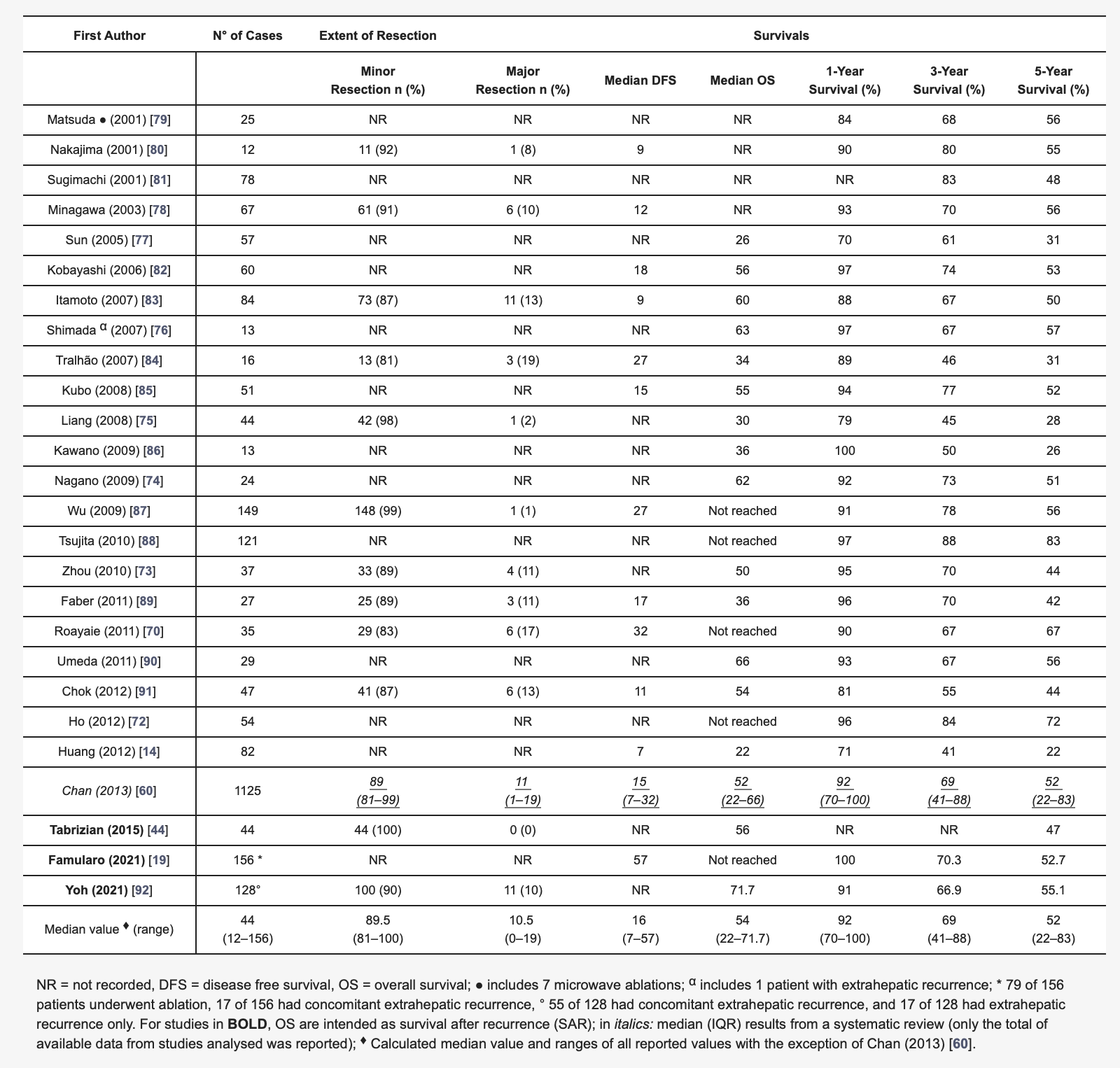
More recent experiences reported median OS after the resection of rHCC of 56 months, and about half of the patients resected alive at 5 years after surgery [44]. Of note, these results are surely an improvement compared to systemic non-curative treatments, which are addressed in a non-selected population [93].
In terms of surgical technique, if redo open liver resection (ROLR) is still regarded as the standard procedure [73][78], redo laparoscopic liver resection (RLLR) has been gaining increasing attention [94][95]. From a systematic review of the literature [96], RLLR has been associated with outcomes comparable to the first LLR [97] and adequate oncological outcome, resulting in no cases of positive surgical margins in the studies analysed [98][99][100][101][102][103] with results to be confirmed by larger sample sizes.
Whenever both ROLR and RLLR are feasible, it is worth noting the removal of tumour-bearing portal territories, that is at the base of all kinds of anatomical resections, reduces the risk of recurrence intended as local IM [104][105][106]. On the other hand, tumour-vessel detachment (R1vasc surgery), which is not in contradiction with the anatomical conduct of the resection, has been proven to be oncologically suitable and may be useful in the event of rHCC [107][108].
Looking at EHR, especially for lung metastases, surgical resection could be indicated as well, but it is reported to be advantageous only when the recurrence is isolated and the patient has had a DFS > 1 year from the initial LR [109].
There is also the role of ablation techniques for liver-only recurrence cases whenever small nodules (<2 cm) or less than three nodules (the largest <3 cm) occur. In these cases, ablation may be regarded as a curative option following the same indications of a first occurrence [16][110]. However, in a large multicentric recent study, radiofrequency ablation resulted in shorter DFS compared to RH, although no differences in OS were reported [111].
4.2. Salvage Liver Transplantation
The standard criteria for liver transplantation in the case of HCC were introduced by Mazzaferro et al. in 1996 [31]. For patients meeting these criteria, the 5-year survival rate was up to 70% with a recurrence rate of less than 15% [112]. About 70% of recurrences are diagnosed within the 2 years following PLT [113], with the lung being the most frequent metastatic site [114][115].
Due to organ shortage, not only the recurrence but the primary HCC is still difficult to approach by PLT in a timely manner. It was from this gap that the work of Majno [20] took place, who estimated that 30% of primary HCC would outgrow the Milan criteria in each 6-month interval (about 5% per month). Following this, they firstly proposed PLR followed by liver transplantation in the case of tumour recurrence or deteriorating liver function, the so-called SLT [20]. After that, the definition of SLT gained several different meanings. SLT could be performed after rHCC following primary resection (as described by Majno) with conceptual difference depending on whether the first HCC was transplantable or not (the so-called “downstaging primary LR”). SLT may also follow PLR before tumour recurrence (“de principe” LT), and the “bridge LT” when PLR is the chosen neoadjuvant treatment before LT [116][117]; however, this attitude had a published experience as a counterpart, showing higher morbidity and mortality of LT after PLR [118]. Additionally, SLT is sometimes performed because of irreversible liver failure after resection (“rescue” LT). Finally, the possibility of performing an SLT following a PLT, when at recurrence, rHCC is still within transplantable criteria, has been raised [119].
Theoretically, rHCC may have a worse biological behaviour; it is still also controversial whether the same transplantation criteria should be applied as for PLT. For this reason, generally approved criteria for SLT are missing, and several efforts have been made looking for the most suitable patient. Accordingly, Zhang et al. [120] compared various existing criteria such as the Milan criteria, the University of California San Francisco criteria (UCSF) [121] and the model for end-stage liver disease (MELD) [122], concluding that the Milan criteria are those associated with the best outcome in the setting of SLT. On the contrary, de Haas et al. [123] proposed a patient with a higher MELD score and no preoperative bridge therapy, such as trans-arterial chemo-emboliation, no postoperative complication after a PLR and low T-stage in the primary resected HCC as the best candidate for SLT. While no agreement exists on the selection criteria for SLT, it is well accepted that the number and the size of rHCC are important factors to be considered during decision making.
Whether a living donor liver transplant (LDLT) rather than a deceased donor liver transplant (DDLT) should be preferred for SLT is still under debate [124]. While no large experiences have been described and no randomized trials have been conducted, specific pros and cons of LDLT and DDLT tested in the case of PLT may be shifted in the direction of SLT [125][126]. In particular, the oncological benefit of LDLT has been argued, since it has been associated with an increased recurrence rate, probably associated with the lack of biological selection made by the test of time [127]. These results are at least controversial, particularly if data reported by a recent multicentric study are considered [128]. In this multicentric intention-to-treat analysis, living donor for PLT resulted to be an independent protective factor for overall death.
If large efforts have been made in analysing recurrence risk after PLT [129][130][131], the lack of data in terms of recurrence after SLT makes comparison challenging. Looking at the PLT experience, significant predictors of recurrence appeared to be the following: poor tumour grading, microvascular invasion and the diameter of the largest tumour [132], with the most favourable prognosis reached in the case of unifocal liver recurrence with concomitant low AFP level [133].
Conflicting results have been reported by the role of PLR preceding transplant (downstaging PLR) since it has been argued that it may represent a possible risk factor for recurrence itself, and for this reason, it should be reserved to limited cases [118][132][134]. However, if bridge liver resection can lead to tumour manipulation, and to longer and more bleeding transplant surgery, it also gives the possibility, throughout the pathological examination of the surgical specimen, to select patients on the basis of the aforementioned risk factors [118][132][134].
Apart from PLR, the role of other bridging therapies has been reported as well. Even if their potential advantages have been analysed mainly in relation to patients awaiting for PLT, such benefits can be translated to patients on the waiting list for a SLT, since no specific guidelines are present. These should be considered as a sort of neoadjuvant treatment, mainly consisting of several types of locoregional procedures (TACE, transarterial radioembolization, stereotactic body radiotherapy), to avoid tumour progression, thus reducing the drop-out rate of patients on the waitlist. For PLT, bridge therapies have been proposed when the expected waiting time on the list exceeds 6 months with a complete response to LRT, resulting in a significant reduction of dropout at 3, 6 and 12 months [135]. On the other hand, as proposed for PLT, an unsatisfactory response to these treatments may represent a criterion for prioritizing patients on the waitlist [136].
4.3. RH vs. SLT: Which Is the Best Option?
Several studies have reported a comparison between SLT and RH in the case of rHCC (Table 2) [119][137][138][139][140]. Clearly, there are many factors to be considered, not least the experience of a given centre in performing both liver resection and transplantation. Apart from the specific centre criteria, the length of the waiting list, the general organ shortage, and the type of LT (LDLT versus DDLT), the patient’s age remains an important factor to be taken into consideration. While there is no longer a given age limit for LT in some liver transplant centres, being old may still represent per se a contraindication for LT and for SLT. For these reasons, RH appears to be more feasible than SLT, in particular for older patients. Moreover, RH could be performed when some prohibitive conditions for liver transplantation (e.g., macrovascular invasion) are present, along with the concept of the “therapeutic hierarchy” as historically endorsed by the Asia-Pacific treatment algorithm [61]. On the other hand, SLT may be considered if unresectable cases present with conditions that still fall in specific centre criteria of transplantability.
Table 2. Studies comparing redo hepatectomy versus salvage liver transplantation.
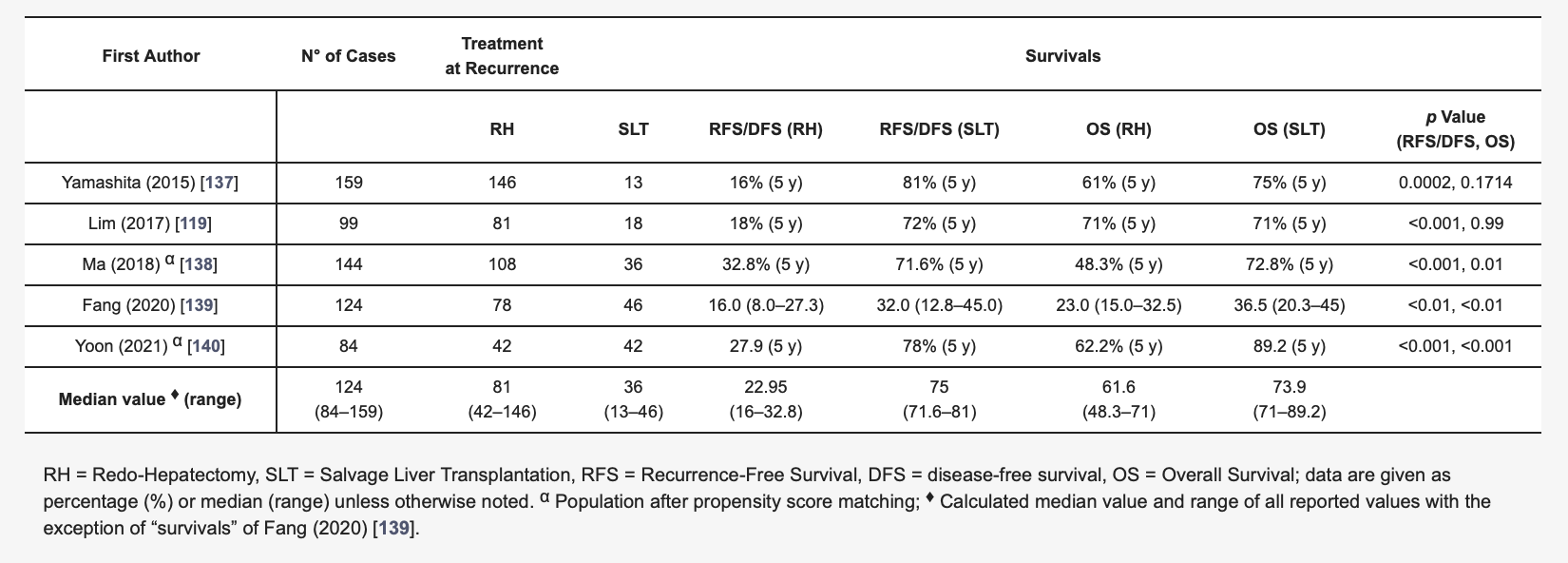
More in detail, when looking at the survival benefit of RH versus SLT, some discrepancies from the review of the literature emerged. In 2020, Fang et al. [138] described their experience with 124 patients undergoing RH or SLT after rHCC following PLR, reporting recurrence-free survival and OS rates that were significantly higher in the SLT group. This was despite the two groups differing in terms of preoperative total bilirubin levels, number of multiple tumours (higher in the SLT group) and HCC size (higher in the RH group). However, the advantage of SLT over RH was not confirmed when only patients with higher AFP at recurrence (>100 ng/mL) were considered, indicating that the selection process for RH versus SLT is complex. Furthermore, Fang et al. [139] reported longer operation time, increased blood loss and blood replacement, prolonged in-hospital stays and postoperative morbidity in the case of SLT; in addition, perioperative mortality was not reported for the groups.
In another HCC patient cohort previously treated with PLR or ablation, Ma et al. [138] reported, with the use of propensity score matching (PSM), that the 5-year OS and tumour-free survival were higher in the SLT group (with no distinction between DDLT and LDLT) versus RH. A similar propensity score matching analysis was also performed by Yoon et al. [140], who focused on a possible benefit of LDLT compared to RH. This resulted in LDLT having a longer DFS than RH even after a PSM. Along this line, some other papers deserve to be mentioned. Wang et al. [40] analysed 840 patients and compared SLT with curative loco-regional therapy (CLRT). The SLT was found to be associated with significantly higher 3- and 5-year DFS than in the CRLT group, but no OS benefit was achieved. SLT resulted in greater intraoperative blood loss and longer in-hospital stays [40]. Kostakis et al. [141] reported a similar analysis on 516 patients. They demonstrated that SLT was burdened by higher rates of postoperative complications and mortality, even if the higher rate of death did not reach statistical significance. Yamashita et al. [137] retrospectively studied the efficacy of SLT (exclusively LDLT) versus RH in a population of 146 patients with rHCC treated by PLR. Once again, no statistically significant differences in OS were found between the two treatments with the SLT showing a longer 5-year DFS rate (86% versus 16%). Lim et al. [119] conducted an intention-to-treat analysis in which the patients collected were previously transplanted due to resectable and transplantable primary and recurrent HCCs. The 90-day mortality was 4% for the SLT group and 0% in the RH (p = 0.007). The 5-year DFS rates were 72% for the SLT and 18% for the RH (p < 0.001). However, at a 5-year intention-to-treat analysis of OS, no statistically significant differences were recorded between SLT and RH [119].
From all these reported experiences, there is need for a clear consensus that can precisely define the proper treatment strategy in cases of rHCC, as performed for primary occurrence disease. The multispecialistic armamentarium from medical, radiological and surgical options should be precisely addressed in a tailored manner on patient characteristics. This highlights the demanding role of a multidisciplinary approach with the multimodality care representing the best solution in the high variability of recurrence presentation. The growing role of histopathologic and genetic prognostic characteristics allows surgical treatment to no longer be a technical problem, and this should be widely discussed in a case-by-case manner. The test of time may represent an underestimated concept, with the IM/MO characterization of recurrence still misunderstood. From the evidence reported and from the evidence coming from the ongoing trials, particularly on immunotherapies strategies, a new important consensus treatment algorithm should be stated.
5. Conclusions
In conclusion, according to our critical review of the literature, it is clear that both RH and SLT are feasible procedures in the setting of rHCC. If SLT consists, as expected, in a more complex procedure burdened by increased postoperative morbidities and mortality, it is also associated with longer DFS. Conversely, RH represents a valid chance of cure, especially for the oldest patients or patients with comorbidities with limited postoperative risks. While waiting for the new and promising immunotherapies in the adjuvant setting, further randomized clinical trials comparing SLT versus RH should be performed in the setting of rHCC.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15020508
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular Carcinoma. Lancet 2003, 362, 1907–1917.
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691.
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1.
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the Epidemic of Nonalcoholic Fatty Liver Disease Demonstrates an Exponential Increase in Burden of Disease. Hepatology 2018, 67, 123–133.
- Belghiti, J.; Kianmanesh, R. Surgical Treatment of Hepatocellular Carcinoma. HPB 2005, 7, 42–49.
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.-N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations?: An Observational Study of the HCC East-West Study Group. Ann. Surg. 2013, 257, 929–937.
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-Analysis of Determinants of Survival Following Treatment of Recurrent Hepatocellular Carcinoma. Br. J. Surg. 2017, 104, 1433–1442.
- Nagasue, N.; Uchida, M.; Makino, Y.; Takemoto, Y.; Yamanoi, A.; Hayashi, T.; Chang, Y.-C.; Kohno, H.; Nakamura, T.; Yukaya, H. Incidence and Factors Associated with Intrahepatic Recurrence Following Resection of Hepatocellular Carcinoma. Gastroenterology 1993, 105, 488–494.
- Vauthey, J.N.; Klimstra, D.; Franceschi, D.; Tao, Y.; Fortner, J.; Blumgart, L.; Brennan, M. Factors Affecting Long-Term Outcome after Hepatic Resection for Hepatocellular Carcinoma. Am. J. Surg. 1995, 169, 28–34; discussion 34–35.
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk Factors Contributing to Early and Late Phase Intrahepatic Recurrence of Hepatocellular Carcinoma after Hepatectomy. J. Hepatol. 2003, 38, 200–207.
- Xie, D.-Y.; Fan, H.-K.; Ren, Z.-G.; Fan, J.; Gao, Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin. Transl. Gastroenterol. 2019, 10, e00006.
- Portolani, N.; Coniglio, A.; Ghidoni, S.; Giovanelli, M.; Benetti, A.; Tiberio, G.A.M.; Giulini, S.M. Early and Late Recurrence after Liver Resection for Hepatocellular Carcinoma: Prognostic and Therapeutic Implications. Ann. Surg. 2006, 243, 229–235.
- Huang, Z.-Y.; Liang, B.-Y.; Xiong, M.; Zhan, D.-Q.; Wei, S.; Wang, G.-P.; Chen, Y.-F.; Chen, X.-P. Long-Term Outcomes of Repeat Hepatic Resection in Patients with Recurrent Hepatocellular Carcinoma and Analysis of Recurrent Types and Their Prognosis: A Single-Center Experience in China. Ann. Surg. Oncol. 2012, 19, 2515–2525.
- Wei, T.; Zhang, X.-F.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; et al. Early Versus Late Recurrence of Hepatocellular Carcinoma After Surgical Resection Based on Post-Recurrence Survival: An International Multi-Institutional Analysis. J. Gastrointest. Surg. 2021, 25, 125–133.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236.
- Akamatsu, N.; Cillo, U.; Cucchetti, A.; Donadon, M.; Pinna, A.D.; Torzilli, G.; Kokudo, N. Surgery and Hepatocellular Carcinoma. Liver Cancer 2016, 6, 44–50.
- Wen, T.; Jin, C.; Facciorusso, A.; Donadon, M.; Han, H.-S.; Mao, Y.; Dai, C.; Cheng, S.; Zhang, B.; Peng, B.; et al. Multidisciplinary Management of Recurrent and Metastatic Hepatocellular Carcinoma after Resection: An International Expert Consensus. Hepatobiliary Surg. Nutr. 2018, 7, 353–371.
- Famularo, S.; Donadon, M.; Cipriani, F.; Bernasconi, D.P.; LaBarba, G.; Dominioni, T.; Iaria, M.; Molfino, S.; Conci, S.; Ferrari, C.; et al. Curative versus Palliative Treatments for Recurrent Hepatocellular Carcinoma: A Multicentric Weighted Comparison. HPB 2021, 23, 889–898.
- Majno, P.E.; Sarasin, F.P.; Mentha, G.; Hadengue, A. Primary Liver Resection and Salvage Transplantation or Primary Liver Transplantation in Patients with Single, Small Hepatocellular Carcinoma and Preserved Liver Function: An Outcome-Oriented Decision Analysis. Hepatology 2000, 31, 899–906.
- Guerrini, G.P.; Esposito, G.; Olivieri, T.; Magistri, P.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers 2022, 14, 3465.
- Sherman, M. Recurrence of Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 2045–2047.
- Famularo, S.; Di Sandro, S.; Giani, A.; Lauterio, A.; Sandini, M.; De Carlis, R.; Buscemi, V.; Uggeri, F.; Romano, F.; Gianotti, L.; et al. Recurrence Patterns After Anatomic or Parenchyma-Sparing Liver Resection for Hepatocarcinoma in a Western Population of Cirrhotic Patients. Ann. Surg. Oncol. 2018, 25, 3974–3981.
- Komuta, M. Histological Heterogeneity of Primary Liver Cancers: Clinical Relevance, Diagnostic Pitfalls and the Pathologist’s Role. Cancers 2021, 13, 2871.
- Sweed, D.; Sweed, E.; Moaz, I.; Mosbeh, A.; Fayed, Y.; Elhamed, S.M.A.; Sweed, E.; Macshut, M.; Abdelsattar, S.; Kilany, S.; et al. The Clinicopathological and Prognostic Factors of Hepatocellular Carcinoma: A 10-Year Tertiary Center Experience in Egypt. World J. Surg. Oncol. 2022, 20, 298.
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological Subtypes of Hepatocellular Carcinoma Are Related to Gene Mutations and Molecular Tumour Classification. J. Hepatol. 2017, 67, 727–738.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188.
- Boyault, S.; Rickman, D.S.; de Reyniès, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Hérault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome Classification of HCC Is Related to Gene Alterations and to New Therapeutic Targets. Hepatology 2007, 45, 42–52.
- Tampaki, M.; Papatheodoridis, G.V.; Cholongitas, E. Intrahepatic Recurrence of Hepatocellular Carcinoma after Resection: An Update. Clin. J. Gastroenterol. 2021, 14, 699–713.
- Andreou, A.; Bahra, M.; Schmelzle, M.; Öllinger, R.; Sucher, R.; Sauer, I.M.; Guel-Klein, S.; Struecker, B.; Eurich, D.; Klein, F.; et al. Predictive Factors for Extrahepatic Recurrence of Hepatocellular Carcinoma Following Liver Transplantation. Clin. Transplant. 2016, 30, 819–827.
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–699.
- Yang, S.-L.; Luo, Y.-Y.; Chen, M.; Zhou, Y.-P.; Lu, F.-R.; Deng, D.-F.; Wu, Y.-R. A Systematic Review and Meta-Analysis Comparing the Prognosis of Multicentric Occurrence and vs. Intrahepatic Metastasis in Patients with Recurrent Hepatocellular Carcinoma after Hepatectomy. HPB 2017, 19, 835–842.
- Carissimi, F.; Barbaglia, M.N.; Salmi, L.; Ciulli, C.; Roccamatisi, L.; Cordaro, G.; Mallela, V.R.; Minisini, R.; Leone, B.E.; Donadon, M.; et al. Finding the Seed of Recurrence: Hepatocellular Carcinoma Circulating Tumor Cells and Their Potential to Drive the Surgical Treatment. World J. Gastrointest. Surg. 2021, 13, 967–978.
- Marubashi, S.; Gotoh, K.; Akita, H.; Takahashi, H.; Sugimura, K.; Miyoshi, N.; Motoori, M.; Kishi, K.; Noura, S.; Fujiwara, Y.; et al. Analysis of Recurrence Patterns After Anatomical or Non-Anatomical Resection for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015, 22, 2243–2252.
- Yamamoto, T.; Kajino, K.; Kudo, M.; Sasaki, Y.; Arakawa, Y.; Hino, O. Determination of the Clonal Origin of Multiple Human Hepatocellular Carcinomas by Cloning and Polymerase Chain Reaction of the Integrated Hepatitis B Virus DNA. Hepatology 1999, 29, 1446–1452.
- Li, Q.; Wang, J.; Juzi, J.T.; Sun, Y.; Zheng, H.; Cui, Y.; Li, H.; Hao, X. Clonality Analysis for Multicentric Origin and Intrahepatic Metastasis in Recurrent and Primary Hepatocellular Carcinoma. J. Gastrointest. Surg. 2008, 12, 1540–1547.
- Sakon, M.; Nagano, H.; Nakamori, S.; Dono, K.; Umeshita, K.; Murakami, T.; Nakamura, H.; Monden, M. Intrahepatic Recurrences of Hepatocellular Carcinoma after Hepatectomy: Analysis Based on Tumor Hemodynamics. Arch. Surg. 2002, 137, 94–99.
- Sakon, M.; Nagano, H.; Shimizu, J.; Kondo, M.; Nakamori, S.; Dono, K.; Umeshita, K.; Nakamura, H.; Murakami, T.; Monden, M. Hepatic Resection of Hepatocellular Carcinomas Based on Tumor Hemodynamics. J. Surg. Oncol. 2000, 73, 179–181.
- Famularo, S.; Piardi, T.; Molfino, S.; Di Martino, M.; Ferrari, C.; Ielpo, B.; Diago, M.V.; Giani, A.; Griseri, G.; Terés, L.B.; et al. Factors Affecting Local and Intra Hepatic Distant Recurrence After Surgery for Hcc: An Alternative Perspective on Microvascular Invasion and Satellitosis—A Western European Multicentre Study. J. Gastrointest. Surg. 2021, 25, 104–111.
- Wang, H.-L.; Mo, D.-C.; Zhong, J.-H.; Ma, L.; Wu, F.-X.; Xiang, B.-D.; Li, L.-Q. Systematic Review of Treatment Strategy for Recurrent Hepatocellular Carcinoma: Salvage Liver Transplantation or Curative Locoregional Therapy. Medicine 2019, 98, e14498.
- Tajima, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shinozaki, K.; Nishie, A.; Honda, H.; Shimada, M. Detecting Postsurgical Recurrent Hepatocellular Carcinoma with Multiphasic Helical Computed Tomography: Intrahepatic Metastasis or Multicentric Occurrence? J. Comput. Assist. Tomogr. 2005, 29, 42–50.
- Nakashima, O.; Kojiro, M. Recurrence of Hepatocellular Carcinoma: Multicentric Occurrence or Intrahepatic Metastasis? A Viewpoint in Terms of Pathology. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 404–409.
- Cucchetti, A.; Piscaglia, F.; Caturelli, E.; Benvegnù, L.; Vivarelli, M.; Ercolani, G.; Cescon, M.; Ravaioli, M.; Grazi, G.L.; Bolondi, L.; et al. Comparison of Recurrence of Hepatocellular Carcinoma after Resection in Patients with Cirrhosis to Its Occurrence in a Surveilled Cirrhotic Population. Ann. Surg. Oncol. 2009, 16, 413–422.
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer after Resection: Patterns, Treatments, and Prognosis. Ann. Surg. 2015, 261, 947–955.
- Ng, I.O.-L.; Guan, X.-Y.; Poon, R.T.-P.; Fan, S.-T.; Lee, J.M.-F. Determination of the Molecular Relationship between Multiple Tumour Nodules in Hepatocellular Carcinoma Differentiates Multicentric Origin from Intrahepatic Metastasis. J. Pathol. 2003, 199, 345–353.
- Niu, Z.-S.; Niu, X.-J.; Wang, W.-H. Genetic Alterations in Hepatocellular Carcinoma: An Update. World J. Gastroenterol. 2016, 22, 9069–9095.
- Furuta, M.; Ueno, M.; Fujimoto, A.; Hayami, S.; Yasukawa, S.; Kojima, F.; Arihiro, K.; Kawakami, Y.; Wardell, C.P.; Shiraishi, Y.; et al. Whole Genome Sequencing Discriminates Hepatocellular Carcinoma with Intrahepatic Metastasis from Multi-Centric Tumors. J. Hepatol. 2017, 66, 363–373.
- Fujiki, M.; Aucejo, F.; Kim, R. Adjuvant Treatment of Hepatocellular Carcinoma after Orthotopic Liver Transplantation: Do We Really Need This? Clin. Transplant. 2013, 27, 169–177.
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant Sorafenib for Hepatocellular Carcinoma after Resection or Ablation (STORM): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 1344–1354.
- Han, B.; Ding, H.; Zhao, S.; Zhang, Y.; Wang, J.; Zhang, Y.; Gu, J. Potential Role of Adjuvant Lenvatinib in Improving Disease-Free Survival for Patients With High-Risk Hepatitis B Virus-Related Hepatocellular Carcinoma Following Liver Transplantation: A Retrospective, Case Control Study. Front. Oncol. 2020, 10, 562103.
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of Tumor-Associated Antigen-Specific T Cell Responses by Radiofrequency Ablation of Hepatocellular Carcinoma. Hepatology 2013, 57, 1448–1457.
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551.
- Kudo, M. Adjuvant Immunotherapy after Curative Treatment for Hepatocellular Carcinoma. Lichenologist 2021, 10, 399–403.
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126.
- Morita, M.; Nishida, N.; Sakai, K.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; et al. Immunological Microenvironment Predicts the Survival of the Patients with Hepatocellular Carcinoma Treated with Anti-PD-1 Antibody. Liver Cancer 2021, 10, 380–393.
- Sun, J.J.; Wang, K.; Zhang, C.Z.; Guo, W.X.; Shi, J.; Cong, W.M.; Wu, M.C.; Lau, W.Y.; Cheng, S.Q. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who Have Hepatocellular Carcinoma with Microvascular Invasion. Ann. Surg. Oncol. 2016, 23, 1344–1351.
- Wang, L.; Wang, W.; Yao, X.; Rong, W.; Wu, F.; Chen, B.; Liu, M.; Lin, S.; Liu, Y.; Wu, J. Postoperative Adjuvant Radiotherapy Is Associated with Improved Survival in Hepatocellular Carcinoma with Microvascular Invasion. Oncotarget 2017, 8, 79971–79981.
- Chen, Z.-H.; Zhang, X.-P.; Zhou, T.-F.; Wang, K.; Wang, H.; Chai, Z.-T.; Shi, J.; Guo, W.-X.; Cheng, S.-Q. Adjuvant Transarterial Chemoembolization Improves Survival Outcomes in Hepatocellular Carcinoma with Microvascular Invasion: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2019, 45, 2188–2196.
- Nagasue, N.; Yukaya, H.; Ogawa, Y.; Sasaki, Y.; Chang, Y.C.; Niimi, K. Second Hepatic Resection for Recurrent Hepatocellular Carcinoma. Br. J. Surg. 1986, 73, 434–438.
- Chan, D.L.; Morris, D.L.; Chua, T.C. Clinical Efficacy and Predictors of Outcomes of Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma—A Systematic Review. Surg. Oncol. 2013, 22, e23–e30.
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol. Int. 2017, 11, 317–370.
- Vitale, A.; Farinati, F.; Noaro, G.; Burra, P.; Pawlik, T.M.; Bucci, L.; Giannini, E.G.; Faggiano, C.; Ciccarese, F.; Rapaccini, G.L.; et al. Restaging Patients With Hepatocellular Carcinoma Before Additional Treatment Decisions: A Multicenter Cohort Study. Hepatology 2018, 68, 1232–1244.
- Child, C.G.; Turcotte, J.G. Surgery and Portal Hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85.
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br. J. Surg. 1973, 60, 646–649.
- Donadon, M.; Costa, G.; Cimino, M.; Procopio, F.; Fabbro, D.D.; Palmisano, A.; Torzilli, G. Safe Hepatectomy Selection Criteria for Hepatocellular Carcinoma Patients: A Validation of 336 Consecutive Hepatectomies. The BILCHE Score. World J. Surg. 2015, 39, 237–243.
- Makuuchi, M.; Kosuge, T.; Takayama, T.; Yamazaki, S.; Kakazu, T.; Miyagawa, S.; Kawasaki, S. Surgery for Small Liver Cancers. Semin. Surg. Oncol. 1993, 9, 298–304.
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC Guidelines) 2019 Update. Hepatol. Res. 2019, 49, 1109–1113.
- Bosch, J.; Abraldes, J.G.; Berzigotti, A.; García-Pagan, J.C. The Clinical Use of HVPG Measurements in Chronic Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 573–582.
- Shubert, C.R.; Habermann, E.B.; Truty, M.J.; Thomsen, K.M.; Kendrick, M.L.; Nagorney, D.M. Defining Perioperative Risk after Hepatectomy Based on Diagnosis and Extent of Resection. J. Gastrointest. Surg. 2014, 18, 1917–1928.
- Roayaie, S.; Bassi, D.; Tarchi, P.; Labow, D.; Schwartz, M. Second Hepatic Resection for Recurrent Hepatocellular Cancer: A Western Experience. J. Hepatol. 2011, 55, 346–350.
- Notake, T.; Kobayashi, A.; Shinkawa, H.; Kawahara, T.; Shimizu, A.; Yokoyama, T.; Hasegawa, K.; Kokudo, N.; Matsuyama, Y.; Makuuchi, M.; et al. Nomogram Predicting Long-Term Survival after the Diagnosis of Intrahepatic Recurrence of Hepatocellular Carcinoma Following an Initial Liver Resection. Int. J. Clin. Oncol. 2017, 22, 715–725.
- Ho, C.-M.; Lee, P.-H.; Shau, W.-Y.; Ho, M.-C.; Wu, Y.-M.; Hu, R.-H. Survival in Patients with Recurrent Hepatocellular Carcinoma after Primary Hepatectomy: Comparative Effectiveness of Treatment Modalities. Surgery 2012, 151, 700–709.
- Zhou, Y.; Sui, C.; Li, B.; Yin, Z.; Tan, Y.; Yang, J.; Liu, Z. Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Local Experience and a Systematic Review. World J. Surg. Oncol. 2010, 8, 55.
- Nagano, Y.; Shimada, H.; Ueda, M.; Matsuo, K.; Tanaka, K.; Endo, I.; Kunisaki, C.; Togo, S. Efficacy of Repeat Hepatic Resection for Recurrent Hepatocellular Carcinomas. ANZ J. Surg. 2009, 79, 729–733.
- Liang, H.-H.; Chen, M.-S.; Peng, Z.-W.; Zhang, Y.-J.; Zhang, Y.-Q.; Li, J.-Q.; Lau, W.Y. Percutaneous Radiofrequency Ablation versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Retrospective Study. Ann. Surg. Oncol. 2008, 15, 3484–3493.
- Shimada, K.; Sakamoto, Y.; Esaki, M.; Kosuge, T.; Morizane, C.; Ikeda, M.; Ueno, H.; Okusaka, T.; Arai, Y.; Takayasu, K. Analysis of Prognostic Factors Affecting Survival after Initial Recurrence and Treatment Efficacy for Recurrence in Patients Undergoing Potentially Curative Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2007, 14, 2337–2347.
- Sun, H.-C.; Tang, Z.-Y.; Ma, Z.-C.; Qin, L.-X.; Wang, L.; Ye, Q.-H.; Fan, J.; Wu, Z.-Q.; Zhou, X.-D. The Prognostic Factor for Outcome Following Second Resection for Intrahepatic Recurrence of Hepatocellular Carcinoma with a Hepatitis B Virus Infection Background. J. Cancer Res. Clin. Oncol. 2005, 131, 284–288.
- Minagawa, M.; Makuuchi, M.; Takayama, T.; Kokudo, N. Selection Criteria for Repeat Hepatectomy in Patients with Recurrent Hepatocellular Carcinoma. Ann. Surg. 2003, 238, 703–710.
- Matsuda, M.; Fujii, H.; Kono, H.; Matsumoto, Y. Surgical Treatment of Recurrent Hepatocellular Carcinoma Based on the Mode of Recurrence: Repeat Hepatic Resection or Ablation Are Good Choices for Patients with Recurrent Multicentric Cancer. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 353–359.
- Nakajima, Y.; Ko, S.; Kanamura, T.; Nagao, M.; Kanehiro, H.; Hisanaga, M.; Aomatsu, Y.; Ikeda, N.; Nakano, H. Repeat Liver Resection for Hepatocellular Carcinoma. J. Am. Coll. Surg. 2001, 192, 339–344.
- Sugimachi, K.; Maehara, S.; Tanaka, S.; Shimada, M.; Sugimachi, K. Repeat Hepatectomy Is the Most Useful Treatment for Recurrent Hepatocellular Carcinoma. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 410–416.
- Kobayashi, A.; Kawasaki, S.; Miyagawa, S.-I.; Miwa, S.; Noike, T.; Takagi, S.; Iijima, S.; Miyagawa, Y. Results of 404 Hepatic Resections Including 80 Repeat Hepatectomies for Hepatocellular Carcinoma. Hepatogastroenterology 2006, 53, 736–741.
- Itamoto, T.; Nakahara, H.; Amano, H.; Kohashi, T.; Ohdan, H.; Tashiro, H.; Asahara, T. Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Surgery 2007, 141, 589–597.
- Tralhão, J.G.; Dagher, I.; Lino, T.; Roudié, J.; Franco, D. Treatment of Tumour Recurrence after Resection of Hepatocellular Carcinoma. Analysis of 97 Consecutive Patients. Eur. J. Surg. Oncol. 2007, 33, 746–751.
- Kubo, S.; Takemura, S.; Uenishi, T.; Yamamoto, T.; Ohba, K.; Ogawa, M.; Hai, S.; Ichikawa, T.; Kodai, S.; Shinkawa, H.; et al. Second Hepatic Resection for Recurrent Hepatocellular Carcinoma in Patients with Chronic Hepatitis C. World J. Surg. 2008, 32, 632–638.
- Kawano, Y.; Sasaki, A.; Kai, S.; Endo, Y.; Iwaki, K.; Uchida, H.; Shibata, K.; Ohta, M.; Kitano, S. Prognosis of Patients with Intrahepatic Recurrence after Hepatic Resection for Hepatocellular Carcinoma: A Retrospective Study. Eur. J. Surg. Oncol. 2009, 35, 174–179.
- Wu, C.-C.; Cheng, S.-B.; Yeh, D.-C.; Wang, J.; P’eng, F.-K. Second and Third Hepatectomies for Recurrent Hepatocellular Carcinoma Are Justified. Br. J. Surg. 2009, 96, 1049–1057.
- Tsujita, E.; Yamashita, Y.-I.; Takeishi, K.; Matsuyama, A.; Tsutsui, S.-I.; Matsuda, H.; Toshima, T.; Taketomi, A.; Shirabe, K.; Ishida, T.; et al. Poor Prognostic Factors after Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma in the Modern Era. Am. Surg. 2012, 78, 419–425.
- Faber, W.; Seehofer, D.; Neuhaus, P.; Stockmann, M.; Denecke, T.; Kalmuk, S.; Warnick, P.; Bahra, M. Repeated Liver Resection for Recurrent Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 1189–1194.
- Umeda, Y.; Matsuda, H.; Sadamori, H.; Matsukawa, H.; Yagi, T.; Fujiwara, T. A Prognostic Model and Treatment Strategy for Intrahepatic Recurrence of Hepatocellular Carcinoma after Curative Resection. World J. Surg. 2011, 35, 170–177.
- Chok, K.S.H.; Chan, S.C.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Re-Resection for Metachronous Primary Hepatocellular Carcinoma: Is It Justified? ANZ J. Surg. 2012, 82, 63–67.
- Yoh, T.; Seo, S.; Taura, K.; Iguchi, K.; Ogiso, S.; Fukumitsu, K.; Ishii, T.; Kaido, T.; Uemoto, S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-Term Survival. Ann. Surg. 2021, 273, 792–799.
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic Treatment of Hepatocellular Carcinoma: An EASL Position Paper. J. Hepatol. 2021, 75, 960–974.
- Ahn, K.S.; Han, H.-S.; Yoon, Y.-S.; Cho, J.Y.; Kim, J.H. Laparoscopic Liver Resection in Patients with a History of Upper Abdominal Surgery. World J. Surg. 2011, 35, 1333–1339.
- Shelat, V.G.; Serin, K.; Samim, M.; Besselink, M.G.; Al Saati, H.; Di Gioia, P.; Pearce, N.W.; Abu Hilal, M. Outcomes of Repeat Laparoscopic Liver Resection Compared to the Primary Resection. World J. Surg. 2014, 38, 3175–3180.
- Machairas, N.; Papaconstantinou, D.; Stamopoulos, P.; Prodromidou, A.; Garoufalia, Z.; Spartalis, E.; Kostakis, I.D.; Sotiropoulos, G.C. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res. 2018, 38, 3181–3186.
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative Short-Term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann. Surg. 2016, 263, 761–777.
- Belli, G.; Cioffi, L.; Fantini, C.; D’Agostino, A.; Russo, G.; Limongelli, P.; Belli, A. Laparoscopic Redo Surgery for Recurrent Hepatocellular Carcinoma in Cirrhotic Patients: Feasibility, Safety, and Results. Surg. Endosc. 2009, 23, 1807–1811.
- Goh, B.K.P.; Teo, J.-Y.; Chan, C.-Y.; Lee, S.-Y.; Cheow, P.-C.; Chung, A.Y.F. Laparoscopic Repeat Liver Resection for Recurrent Hepatocellular Carcinoma. ANZ J. Surg. 2017, 87, E143–E146.
- Zhang, J.; Zhou, Z.-G.; Huang, Z.-X.; Yang, K.-L.; Chen, J.-C.; Chen, J.-B.; Xu, L.; Chen, M.-S.; Zhang, Y.-J. Prospective, Single-Center Cohort Study Analyzing the Efficacy of Complete Laparoscopic Resection on Recurrent Hepatocellular Carcinoma. Chin. J. Cancer 2016, 35, 25.
- Kim, G.; Lau, A.C.-H.; Chang, S.K.Y. Single-Incision Laparoscopic Hepatic Resection in Patients with Previous Hepatic Resections: A Mini Case Series. Asian J. Endosc. Surg. 2014, 7, 63–66.
- Chan, A.C.Y.; Poon, R.T.P.; Chok, K.S.H.; Cheung, T.T.; Chan, S.C.; Lo, C.M. Feasibility of Laparoscopic Re-Resection for Patients with Recurrent Hepatocellular Carcinoma. World J. Surg. 2014, 38, 1141–1146.
- Liu, K.; Chen, Y.; Wu, X.; Huang, Z.; Lin, Z.; Jiang, J.; Tan, W.; Zhang, L. Laparoscopic Liver Re-Resection Is Feasible for Patients with Posthepatectomy Hepatocellular Carcinoma Recurrence: A Propensity Score Matching Study. Surg. Endosc. 2017, 31, 4790–4798.
- Torzilli, G.; Procopio, F.; Cimino, M.; Del Fabbro, D.; Palmisano, A.; Donadon, M.; Montorsi, M. Anatomical Segmental and Subsegmental Resection of the Liver for Hepatocellular Carcinoma: A New Approach by Means of Ultrasound-Guided Vessel Compression. Ann. Surg. 2010, 251, 229–235.
- Viganò, L.; Procopio, F.; Mimmo, A.; Donadon, M.; Terrone, A.; Cimino, M.; Fabbro, D.D.; Torzilli, G. Oncologic Superiority of Anatomic Resection of Hepatocellular Carcinoma by Ultrasound-Guided Compression of the Portal Tributaries Compared with Nonanatomic Resection: An Analysis of Patients Matched for Tumor Characteristics and Liver Function. Surgery 2018, 164, 1006–1013.
- Shindoh, J.; Makuuchi, M.; Matsuyama, Y.; Mise, Y.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Complete Removal of the Tumor-Bearing Portal Territory Decreases Local Tumor Recurrence and Improves Disease-Specific Survival of Patients with Hepatocellular Carcinoma. J. Hepatol. 2016, 64, 594–600.
- Torzilli, G.; Montorsi, M.; Donadon, M.; Palmisano, A.; Del Fabbro, D.; Gambetti, A.; Olivari, N.; Makuuchi, M. “Radical but Conservative” Is the Main Goal for Ultrasonography-Guided Liver Resection: Prospective Validation of This Approach. J. Am. Coll. Surg. 2005, 201, 517.
- Is R1 Vascular Hepatectomy for Hepatocellular Carcinoma Oncologically Adequate? Analysis of 327 Consecutive Patients. Surgery 2019, 165, 897–904.
- Ohba, T.; Yano, T.; Yoshida, T.; Kawano, D.; Tsukamoto, S.; Shoji, F.; Taketomi, A.; Saitsu, H.; Takeo, S.; Maehara, Y. Results of a Surgical Resection of Pulmonary Metastasis from Hepatocellular Carcinoma: Prognostic Impact of the Preoperative Serum Alpha-Fetoprotein Level. Surg. Today 2012, 42, 526–531.
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380.
- Zhong, J.-H.; Xing, B.-C.; Zhang, W.-G.; Chan, A.W.-H.; Chong, C.C.N.; Serenari, M.; Peng, N.; Huang, T.; Lu, S.-D.; Liang, Z.-Y.; et al. Repeat Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma: Retrospective Multicentre Study. Br. J. Surg. 2021, 109, 71–78.
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314.
- Fernandez-Sevilla, E.; Allard, M.-A.; Selten, J.; Golse, N.; Vibert, E.; Sa Cunha, A.; Cherqui, D.; Castaing, D.; Adam, R. Recurrence of Hepatocellular Carcinoma after Liver Transplantation: Is There a Place for Resection? Liver Transpl. 2017, 23, 440–447.
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118–125.
- Toso, C.; Cader, S.; Mentha-Dugerdil, A.; Meeberg, G.; Majno, P.; Morard, I.; Giostra, E.; Berney, T.; Morel, P.; Mentha, G.; et al. Factors Predicting Survival after Post-Transplant Hepatocellular Carcinoma Recurrence. J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 342–347.
- Belghiti, J.; Cortes, A.; Abdalla, E.K.; Régimbeau, J.-M.; Prakash, K.; Durand, F.; Sommacale, D.; Dondero, F.; Lesurtel, M.; Sauvanet, A.; et al. Resection prior to Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. 2003, 238, 885–892; discussion 892–893.
- Tribillon, E.; Barbier, L.; Goumard, C.; Irtan, S.; Perdigao-Cotta, F.; Durand, F.; Paradis, V.; Belghiti, J.; Scatton, O.; Soubrane, O. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J. Gastrointest. Surg. 2016, 20, 66–76; discussion 76.
- Adam, R.; Azoulay, D.; Castaing, D.; Eshkenazy, R.; Pascal, G.; Hashizume, K.; Samuel, D.; Bismuth, H. Liver Resection as a Bridge to Transplantation for Hepatocellular Carcinoma on Cirrhosis: A Reasonable Strategy? Ann. Surg. 2003, 238, 508–518; discussion 518–519.
- Lim, C.; Shinkawa, H.; Hasegawa, K.; Bhangui, P.; Salloum, C.; Gomez Gavara, C.; Lahat, E.; Omichi, K.; Arita, J.; Sakamoto, Y.; et al. Salvage Liver Transplantation or Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: An Intent-to-Treat Analysis. Liver Transpl. 2017, 23, 1553–1563.
- Zhang, H.-M.; Jiang, W.-T.; Pan, C.; Deng, Y.-L.; Zheng, H.; Shen, Z.-Y. Milan Criteria, University of California, San Francisco, Criteria, and Model for End-Stage Liver Disease Score as Predictors of Salvage Liver Transplantation. Transplant. Proc. 2015, 47, 438–444.
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403.
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A Model to Predict Survival in Patients with End-Stage Liver Disease. Hepatology 2001, 33, 464–470.
- de Haas, R.J.; Lim, C.; Bhangui, P.; Salloum, C.; Compagnon, P.; Feray, C.; Calderaro, J.; Luciani, A.; Azoulay, D. Curative Salvage Liver Transplantation in Patients with Cirrhosis and Hepatocellular Carcinoma: An Intention-to-Treat Analysis. Hepatology 2018, 67, 204–215.
- Kaido, T.; Uemoto, S. Does Living Donation Have Advantages over Deceased Donation in Liver Transplantation? J. Gastroenterol. Hepatol. 2010, 25, 1598–1603.
- Lo, C.-M.; Fan, S.T.; Liu, C.L.; Yong, B.H.; Wong, Y.; Lau, G.K.; Lai, C.L.; Ng, I.O.; Wong, J. Lessons Learned from One Hundred Right Lobe Living Donor Liver Transplants. Ann. Surg. 2004, 240, 151–158.
- Lee, S.-G.; Song, G.-W.; Yoon, Y.-I. An Exceptional Series: 5000 Living Donor Liver Transplantations at Asan Medical Center, Seoul, Korea. Transplantation 2019, 103, 1739–1741.
- Zhang, H.-M.; Shi, Y.-X.; Sun, L.-Y.; Zhu, Z.-J. Hepatocellular Carcinoma Recurrence in Living and Deceased Donor Liver Transplantation: A Systematic Review and Meta-Analysis. Chin. Med. J. 2019, 132, 1599–1609.
- Lai, Q.; Sapisochin, G.; Gorgen, A.; Vitale, A.; Halazun, K.J.; Iesari, S.; Schaefer, B.; Bhangui, P.; Mennini, G.; Wong, T.C.L.; et al. Evaluation of the Intention-to-Treat Benefit of Living Donation in Patients With Hepatocellular Carcinoma Awaiting a Liver Transplant. JAMA Surg. 2021, 156, e213112.
- Lo, C.-M.; Fan, S.-T.; Liu, C.-L.; Chan, S.-C.; Wong, J. The Role and Limitation of Living Donor Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2004, 10, 440–447.
- Mak, K.S.W.; Tan, K.C. Liver Transplantation for Hepatocellular Carcinoma: An Asian Perspective. Asian J. Surg. 2002, 25, 271–276.
- Fisher, R.A.; Kulik, L.M.; Freise, C.E.; Lok, A.S.F.; Shearon, T.H.; Brown, R.S., Jr.; Ghobrial, R.M.; Fair, J.H.; Olthoff, K.M.; Kam, I.; et al. Hepatocellular Carcinoma Recurrence and Death Following Living and Deceased Donor Liver Transplantation. Am. J. Transplant 2007, 7, 1601–1608.
- Lai, Q.; Avolio, A.W.; Lerut, J.; Singh, G.; Chan, S.C.; Berloco, P.B.; Tisone, G.; Agnes, S.; Chok, K.S.; Sharr, W.; et al. Recurrence of Hepatocellular Cancer after Liver Transplantation: The Role of Primary Resection and Salvage Transplantation in East and West. J. Hepatol. 2012, 57, 974–979.
- Sapisochin, G.; Goldaracena, N.; Astete, S.; Laurence, J.M.; Davidson, D.; Rafael, E.; Castells, L.; Sandroussi, C.; Bilbao, I.; Dopazo, C.; et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann. Surg. Oncol. 2015, 22, 2286–2294.
- Poon, R.T.-P.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-Term Survival and Pattern of Recurrence after Resection of Small Hepatocellular Carcinoma in Patients with Preserved Liver Function: Implications for a Strategy of Salvage Transplantation. Ann. Surg. 2002, 235, 373–382.
- Cucchetti, A.; Cescon, M.; Bigonzi, E.; Piscaglia, F.; Golfieri, R.; Ercolani, G.; Cristina Morelli, M.; Ravaioli, M.; Daniele Pinna, A. Priority of Candidates with Hepatocellular Carcinoma Awaiting Liver Transplantation Can Be Reduced after Successful Bridge Therapy. Liver Transpl. 2011, 17, 1344–1354.
- Vitale, A.; D’Amico, F.; Frigo, A.C.; Grigoletto, F.; Brolese, A.; Zanus, G.; Neri, D.; Carraro, A.; D’Amico, F.E.; Burra, P.; et al. Response to Therapy as a Criterion for Awarding Priority to Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation. Ann. Surg. Oncol. 2010, 17, 2290–2302.
- Yamashita, Y.-I.; Yoshida, Y.; Kurihara, T.; Itoh, S.; Harimoto, N.; Ikegami, T.; Yoshizumi, T.; Uchiyama, H.; Shirabe, K.; Maehara, Y. Surgical Results for Recurrent Hepatocellular Carcinoma after Curative Hepatectomy: Repeat Hepatectomy versus Salvage Living Donor Liver Transplantation. Liver Transpl. 2015, 21, 961–968.
- Ma, K.W.; Chok, K.S.H.; She, W.H.; Chan, A.C.Y.; Cheung, T.T.; Dai, W.C.; Fung, J.Y.Y.; Lo, C.M. Defining Optimal Surgical Treatment for Recurrent Hepatocellular Carcinoma: A Propensity Score Matched Analysis. Liver Transpl. 2018, 24, 1062–1069.
- Fang, J.-Z.; Xiang, L.; Hu, Y.-K.; Yang, Y.; Zhu, H.-D.; Lu, C.-D. Options for the Treatment of Intrahepatic Recurrent Hepatocellular Carcinoma: Salvage Liver Transplantation or Rehepatectomy? Clin. Transplant. 2020, 34, e13831.
- Yoon, Y.-I.; Song, G.-W.; Lee, S.; Moon, D.; Hwang, S.; Kang, W.-H.; Cho, H.-D.; Ha, S.-M.; Kim, M.-J.; Kim, S.-H.; et al. Salvage Living Donor Liver Transplantation versus Repeat Liver Resection for Patients with Recurrent Hepatocellular Carcinoma and Child-Pugh Class A Liver Cirrhosis: A Propensity Score-Matched Comparison. Am. J. Transplant 2022, 22, 165–176.
- Kostakis, I.D.; Machairas, N.; Prodromidou, A.; Stamopoulos, P.; Garoufalia, Z.; Fouzas, I.; Sotiropoulos, G.C. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Transplant. Proc. 2019, 51, 433–436.
This entry is offline, you can click here to edit this entry!
