Various factors have been linked to abnormal metabolic reprogramming, including gene mutations, epigenetic modifications, altered protein epitopes, and their involvement in the development of disease, including cancer. The presence of multiple distinct hallmarks and the resulting cellular reprogramming process have gradually revealed that these metabolism-related molecules may be able to be used to track or prevent the progression of cancer. Consequently, translational medicines have been developed using metabolic substrates, precursors, and other products depending on their biochemical mechanism of action. It is important to note that these metabolic analogs can also be used for imaging and therapeutic purposes in addition to competing for metabolic functions. In particular, due to their isotopic labeling, these compounds may also be used to localize and visualize tumor cells after uptake.
- cancer metabolism
- metabolic reprogramming
- molecular imaging
- cellular uptake
1. Introduction
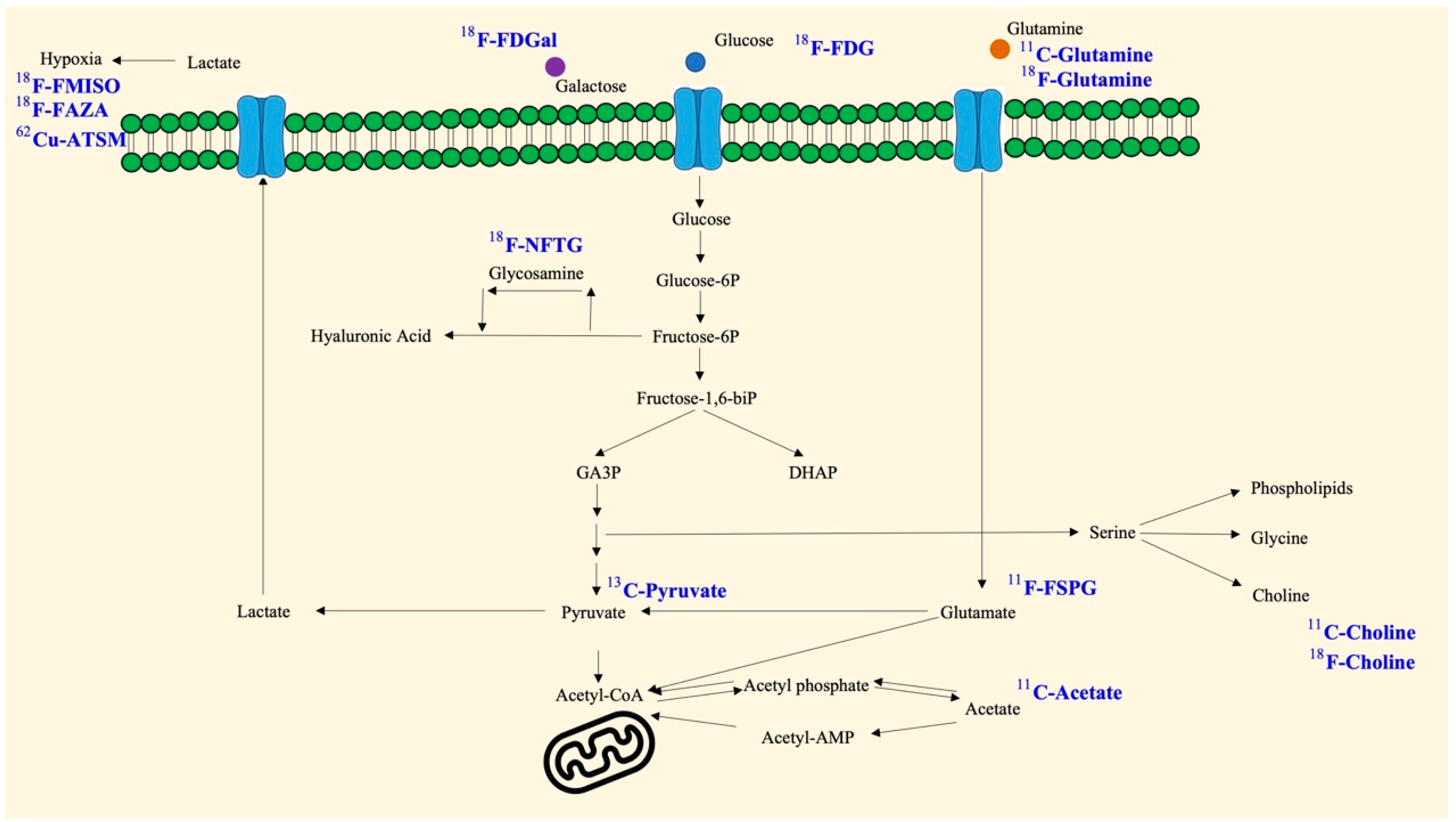
2. Glucose
3. Pyruvate
4. Galactose
5. Choline
6. Acetate
7. Pivalic Acid
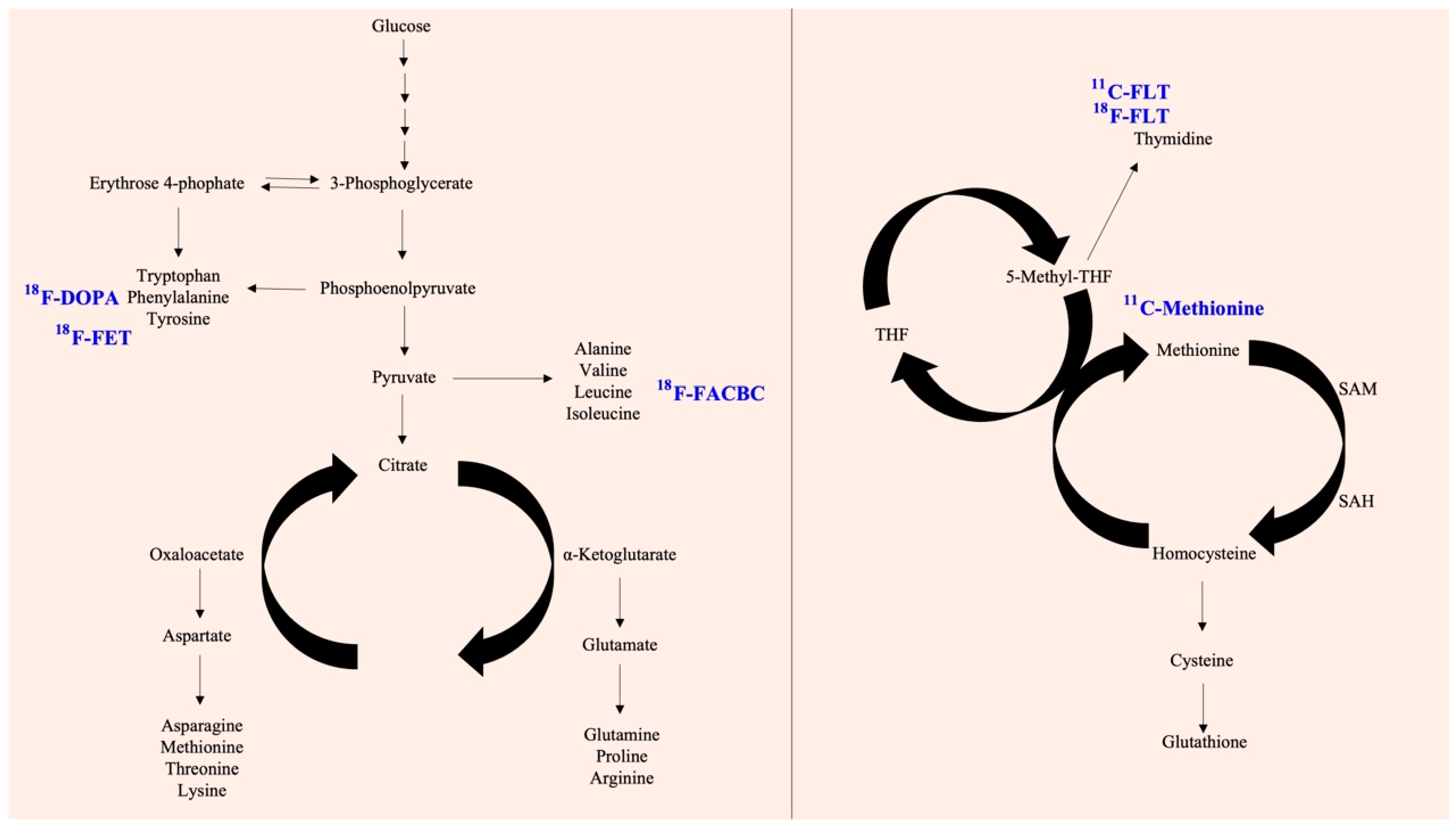
8. Cyclobutanecarboxylic Acid
9. Methionine
10. Glutamine
11. Fluoropropyl-L-Glutamic Acid (FSPG)
12. L-Tyrosine
13. Thymidine
14. Dihydroxyphenylalanine (DOPA)
15. Glucosamine
This entry is adapted from the peer-reviewed paper 10.3390/ijms232415831
References
- Aroldi, F.; Lord, S.R. Window of opportunity clinical trial designs to study cancer metabolism. Br. J. Cancer 2020, 122, 45–51.
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943.
- Kim, Y.H.; Jeong, D.C.; Pak, K.; Han, M.E.; Kim, J.Y.; Liangwen, L.; Kim, H.J.; Kim, T.W.; Kim, T.H.; Hyun, D.W.; et al. SLC2A2 (GLUT2) as a novel prognostic factor for hepatocellular carcinoma. Oncotarget 2017, 8, 68381–68392.
- Chai, Y.J.; Yi, J.W.; Oh, S.W.; Kim, Y.A.; Yi, K.H.; Kim, J.H.; Lee, K.E. Upregulation of SLC2 (GLUT) family genes is related to poor survival outcomes in papillary thyroid carcinoma: Analysis of data from The Cancer Genome Atlas. Surgery 2017, 161, 188–194.
- Flavahan, W.A.; Wu, Q.; Hitomi, M.; Rahim, N.; Kim, Y.; Sloan, A.E.; Weil, R.J.; Nakano, I.; Sarkaria, J.N.; Stringer, B.W.; et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat. Neurosci. 2013, 16, 1373–1382.
- Han, A.L.; Veeneman, B.A.; El-Sawy, L.; Day, K.C.; Day, M.L.; Tomlins, S.A.; Keller, E.T. Fibulin-3 promotes muscle-invasive bladder cancer. Oncogene 2017, 36, 5243–5251.
- Lord, S.R.; Cheng, W.C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K.; et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018, 28, 679–688.e674.
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464.
- Cox, B.L.; Mackie, T.R.; Eliceiri, K.W. The sweet spot: FDG and other 2-carbon glucose analogs for multi-modal metabolic imaging of tumor metabolism. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 1–13.
- Gallamini, A.; Zwarthoed, C.; Borra, A. Positron Emission Tomography (PET) in Oncology. Cancers 2014, 6, 1821–1889.
- Mirus, M.; Tokalov, S.V.; Abramyuk, A.; Heinold, J.; Prochnow, V.; Zöphel, K.; Kotzerke, J.; Abolmaali, N. Noninvasive assessment and quantification of tumor vascularization using FDG-PET/CT and CE-CT in a tumor model with modifiable angiogenesis-an animal experimental prospective cohort study. EJNMMI Res. 2019, 9, 55.
- Namavari, M.; Cheng, Z.; Zhang, R.; De, A.; Levi, J.; Hoerner, J.K.; Yaghoubi, S.S.; Syud, F.A.; Gambhir, S.S. A novel method for direct site-specific radiolabeling of peptides using FDG. Bioconjug. Chem. 2009, 20, 432–436.
- Şenışık, A.M.; İçhedef, Ç.; Kılçar, A.Y.; Uçar, E.; Arı, K.; Göksoy, D.; Parlak, Y.; Sayıt Bilgin, B.E.; Teksöz, S. One-step conjugation of glycylglycine with FDG and a pilot PET imaging study. J. Radioanal. Nucl. Chem. 2018, 316, 457–463.
- Sprinz, C.; Altmayer, S.; Zanon, M.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS ONE 2018, 13, e0193140.
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354.
- Lee, T.C.; Alessio, A.M.; Miyaoka, R.M.; Kinahan, P.E. Morphology supporting function: Attenuation correction for SPECT/CT, PET/CT, and PET/MR imaging. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 25–39.
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143.
- Kim, S.H.; Baek, K.H. Regulation of Cancer Metabolism by Deubiquitinating Enzymes: The Warburg Effect. Int. J. Mol. Sci. 2021, 22, 6173.
- Witney, T.H.; Kettunen, M.I.; Day, S.E.; Hu, D.E.; Neves, A.A.; Gallagher, F.A.; Fulton, S.M.; Brindle, K.M. A comparison between radiolabeled fluorodeoxyglucose uptake and hyperpolarized (13)C-labeled pyruvate utilization as methods for detecting tumor response to treatment. Neoplasia 2009, 11, 574–582, 571 p following 582.
- Serrao, E.M.; Kettunen, M.I.; Rodrigues, T.B.; Lewis, D.Y.; Gallagher, F.A.; Hu, D.E.; Brindle, K.M. Analysis of (13) C and (14) C labeling in pyruvate and lactate in tumor and blood of lymphoma-bearing mice injected with (13) C- and (14) C-labeled pyruvate. NMR Biomed. 2018, 31, e3901.
- Park, J.W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.I.; Lee, W.J.; Kim, C.M.; Nam, B.H. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008, 49, 1912–1921.
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607.
- Sørensen, M.; Frisch, K.; Bender, D.; Keiding, S. The potential use of 2-fluoro-2-deoxy-D-galactose as a PET/CT tracer for detection of hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1723–1731.
- Sørensen, M. Determination of hepatic galactose elimination capacity using 2-fluoro-2-deoxy-D-galactose PET/CT: Reproducibility of the method and metabolic heterogeneity in a normal pig liver model. Scand. J. Gastroenterol. 2011, 46, 98–103.
- Sørensen, M.; Munk, O.L.; Mortensen, F.V.; Olsen, A.K.; Bender, D.; Bass, L.; Keiding, S. Hepatic uptake and metabolism of galactose can be quantified in vivo by 2-fluoro-2-deoxygalactose positron emission tomography. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G27–G36.
- Frisch, K.; Bender, D.; Hansen, S.B.; Keiding, S.; Sørensen, M. Nucleophilic radiosynthesis of 2-fluoro-2-deoxy-D-galactose from Talose triflate and biodistribution in a porcine model. Nucl. Med. Biol. 2011, 38, 477–483.
- Horsager, J.; Bak-Fredslund, K.; Larsen, L.P.; Villadsen, G.E.; Bogsrud, T.V.; Sørensen, M. Optimal 2-fluoro-2-deoxy-D-galactose PET/CT protocol for detection of hepatocellular carcinoma. EJNMMI Res. 2016, 6, 56.
- Bak-Fredslund, K.P.; Munk, O.L.; Keiding, S.; Sørensen, M. 2-fluoro-2-deoxy-D-galactose PET/CT of hepatocellular carcinoma is not improved by co-administration of galactose. Nucl. Med. Biol. 2016, 43, 577–580.
- Spadaro, F.; Ramoni, C.; Mezzanzanica, D.; Miotti, S.; Alberti, P.; Cecchetti, S.; Iorio, E.; Dolo, V.; Canevari, S.; Podo, F. Phosphatidylcholine-specific phospholipase C activation in epithelial ovarian cancer cells. Cancer Res. 2008, 68, 6541–6549.
- Iorio, E.; Ricci, A.; Bagnoli, M.; Pisanu, M.E.; Castellano, G.; Di Vito, M.; Venturini, E.; Glunde, K.; Bhujwalla, Z.M.; Mezzanzanica, D.; et al. Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res. 2010, 70, 2126–2135.
- Kwee, S.A.; Sato, M.M.; Kuang, Y.; Franke, A.; Custer, L.; Miyazaki, K.; Wong, L.L. Fluorocholine PET/CT Imaging of Liver Cancer: Radiopathologic Correlation with Tissue Phospholipid Profiling. Mol. Imaging Biol. 2017, 19, 446–455.
- Kwee, S.A.; Wong, L.; Chan, O.T.M.; Kalathil, S.; Tsai, N. PET/CT with (18)F Fluorocholine as an Imaging Biomarker for Chronic Liver Disease: A Preliminary Radiopathologic Correspondence Study in Patients with Liver Cancer. Radiology 2018, 287, 294–302.
- Wenz, J.; Arndt, F.; Samnick, S. A new concept for the production of (11)C-labelled radiotracers. EJNMMI Radiopharm. Chem. 2022, 7, 6.
- Caribé, P.; Vandenberghe, S.; Diogo, A.; Pérez-Benito, D.; Efthimiou, N.; Thyssen, C.; D’Asseler, Y.; Koole, M. Monte Carlo Simulations of the GE Signa PET/MR for Different Radioisotopes. Front. Physiol. 2020, 11, 525575.
- Boutzios, G.; Sarlanis, H.; Kolindou, A.; Velidaki, A.; Karatzas, T. Primary hyperparathyroidism caused by enormous unilateral water-clear cell parathyroid hyperplasia. BMC Endocr. Disord. 2017, 17, 57.
- Liu, Y.; Dang, Y.; Huo, L.; Hu, Y.; Wang, O.; Liu, H.; Chang, X.; Liu, Y.; Xing, X.; Li, F.; et al. Preoperative Localization of Adenomas in Primary Hyperparathyroidism: The Value of (11)C-Choline PET/CT in Patients with Negative or Discordant Findings on Ultrasonography and (99m)Tc-Sestamibi SPECT/CT. J. Nucl. Med. 2020, 61, 584–589.
- Noltes, M.E.; Kruijff, S.; Jansen, L.; Westerlaan, H.E.; Zandee, W.T.; Dierckx, R.; Brouwers, A.H. A retrospective analysis of the diagnostic performance of (11)C-choline PET/CT for detection of hyperfunctioning parathyroid glands after prior negative or discordant imaging in primary hyperparathyroidism. EJNMMI Res. 2021, 11, 32.
- Welle, C.L.; Cullen, E.L.; Peller, P.J.; Lowe, V.J.; Murphy, R.C.; Johnson, G.B.; Binkovitz, L.A. 11C-Choline PET/CT in Recurrent Prostate Cancer and Nonprostatic Neoplastic Processes. Radiographics 2016, 36, 279–292.
- Krause, B.J.; Souvatzoglou, M.; Tuncel, M.; Herrmann, K.; Buck, A.K.; Praus, C.; Schuster, T.; Geinitz, H.; Treiber, U.; Schwaiger, M. The detection rate of choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 18–23.
- Picchio, M.; Castellucci, P. Clinical Indications of C-Choline PET/CT in Prostate Cancer Patients with Biochemical Relapse. Theranostics 2012, 2, 313–317.
- Bouchelouche, K.; Tagawa, S.T.; Goldsmith, S.J.; Turkbey, B.; Capala, J.; Choyke, P. PET/CT Imaging and Radioimmunotherapy of Prostate Cancer. Semin. Nucl. Med. 2011, 41, 29–44.
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703.
- Schug, Z.T.; Vande Voorde, J.; Gottlieb, E. The metabolic fate of acetate in cancer. Nat. Rev. Cancer 2016, 16, 708–717.
- Ling, R.; Chen, G.; Tang, X.; Liu, N.; Zhou, Y.; Chen, D. Acetyl-CoA synthetase 2(ACSS2): A review with a focus on metabolism and tumor development. Discov. Oncol. 2022, 13, 58.
- Liu, M.; Liu, N.; Wang, J.; Fu, S.; Wang, X.; Chen, D. Acetyl-CoA Synthetase 2 as a Therapeutic Target in Tumor Metabolism. Cancers 2022, 14, 2896.
- Zhou, Z.; Ren, Y.; Yang, J.; Liu, M.; Shi, X.; Luo, W.; Fung, K.M.; Xu, C.; Bronze, M.S.; Zhang, Y.; et al. Acetyl-Coenzyme A Synthetase 2 Potentiates Macropinocytosis and Muscle Wasting Through Metabolic Reprogramming in Pancreatic Cancer. Gastroenterology 2022, 163, 1281–1293.e1281.
- Schug, Z.T.; Peck, B.; Jones, D.T.; Zhang, Q.; Grosskurth, S.; Alam, I.S.; Goodwin, L.M.; Smethurst, E.; Mason, S.; Blyth, K.; et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015, 27, 57–71.
- Shreve, P.; Chiao, P.C.; Humes, H.D.; Schwaiger, M.; Gross, M.D. Carbon-11-acetate PET imaging in renal disease. J. Nucl. Med. 1995, 36, 1595–1601.
- Spick, C.; Herrmann, K.; Czernin, J. Evaluation of Prostate Cancer with 11C-Acetate PET/CT. J. Nucl. Med. 2016, 57, 30s–37s.
- Vees, H.; Buchegger, F.; Albrecht, S.; Khan, H.; Husarik, D.; Zaidi, H.; Soloviev, D.; Hany, T.F.; Miralbell, R. 18F-choline and/or 11C-acetate positron emission tomography: Detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int. 2007, 99, 1415–1420.
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180.
- Brass, E.P. Pivalate-generating prodrugs and carnitine homeostasis in man. Pharmacol. Rev. 2002, 54, 589–598.
- Kuka, J.; Makrecka, M.; Grinberga, S.; Pugovics, O.; Liepinsh, E.; Dambrova, M. A short-term high-dose administration of sodium pivalate impairs pyruvate metabolism without affecting cardiac function. Cardiovasc. Toxicol. 2012, 12, 298–303.
- Dubash, S.R.; Keat, N.; Kozlowski, K.; Barnes, C.; Allott, L.; Brickute, D.; Hill, S.; Huiban, M.; Barwick, T.D.; Kenny, L.; et al. Clinical translation of (18)F-fluoropivalate—A PET tracer for imaging short-chain fatty acid metabolism: Safety, biodistribution, and dosimetry in fed and fasted healthy volunteers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2549–2561.
- Bin, X.; Yong, S.; Kong, Q.F.; Zhao, S.; Zhang, G.Y.; Wu, J.P.; Chen, S.Q.; Zhu, W.D.; Pan, K.H.; Du, M.L.; et al. Diagnostic Performance of PET/CT Using 18F-FACBC in Prostate Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 1438.
- Farkas, A.B.; Green, E.D.; Thaggard, A.L.; Vijayakumar, V.; Henegan, J.C.; Lirette, S.T.; Nittala, M.R.; Vijayakumar, S. Initial Institutional Experience with 18F-Fluciclovine PET-CT in Biochemical Recurrence of Prostate Cancer. South Med. J. 2021, 114, 703–707.
- Wang, K.; Su, R.; Sun, X.; Jiang, J.; Ma, Q. Progress in applications of (18)F-fluciclovine in diagnosis of prostate cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 187–192.
- Schuster, D.M.; Savir-Baruch, B.; Nieh, P.T.; Master, V.A.; Halkar, R.K.; Rossi, P.J.; Lewis, M.M.; Nye, J.A.; Yu, W.; Bowman, F.D.; et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology 2011, 259, 852–861.
- Movahedi, P.; Merisaari, H.; Perez, I.M.; Taimen, P.; Kemppainen, J.; Kuisma, A.; Eskola, O.; Teuho, J.; Saunavaara, J.; Pesola, M.; et al. Prediction of prostate cancer aggressiveness using (18)F-Fluciclovine (FACBC) PET and multisequence multiparametric MRI. Sci. Rep. 2020, 10, 9407.
- Alberts, I.L.; Seide, S.E.; Mingels, C.; Bohn, K.P.; Shi, K.; Zacho, H.D.; Rominger, A.; Afshar-Oromieh, A. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: A systematic review and network meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989.
- Hayashi, T.; Teruya, T.; Chaleckis, R.; Morigasaki, S.; Yanagida, M. S-Adenosylmethionine Synthetase Is Required for Cell Growth, Maintenance of G0 Phase, and Termination of Quiescence in Fission Yeast. iScience 2018, 5, 38–51.
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine metabolism in health and cancer: A nexus of diet and precision medicine. Nat. Rev. Cancer 2019, 19, 625–637.
- Hoffman, R.M. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: A 40-year odyssey. Expert Opin. Biol. Ther. 2015, 15, 21–31.
- Cavuoto, P.; Fenech, M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012, 38, 726–736.
- Jeon, H.; Kim, J.H.; Lee, E.; Jang, Y.J.; Son, J.E.; Kwon, J.Y.; Lim, T.G.; Kim, S.; Park, J.H.; Kim, J.E.; et al. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget 2016, 7, 67223–67234.
- Sun, A.; Liu, X.; Tang, G. Carbon-11 and Fluorine-18 Labeled Amino Acid Tracers for Positron Emission Tomography Imaging of Tumors. Front. Chem. 2017, 5, 124.
- Glaudemans, A.W.; Enting, R.H.; Heesters, M.A.; Dierckx, R.A.; van Rheenen, R.W.; Walenkamp, A.M.; Slart, R.H. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 615–635.
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: Radiomics approach with random forest classifier. Sci. Rep. 2019, 9, 15666.
- Nakajima, R.; Kimura, K.; Abe, K.; Sakai, S. (11)C-methionine PET/CT findings in benign brain disease. Jpn. J. Radiol. 2017, 35, 279–288.
- Nakajo, K.; Uda, T.; Kawashima, T.; Terakawa, Y.; Ishibashi, K.; Tsuyuguchi, N.; Tanoue, Y.; Nagahama, A.; Uda, H.; Koh, S.; et al. Maximum 11C-methionine PET uptake as a prognostic imaging biomarker for newly diagnosed and untreated astrocytic glioma. Sci. Rep. 2022, 12, 546.
- Wang, Y.; Rapalino, O.; Heidari, P.; Loeffler, J.; Shih, H.A.; Oh, K.; Mahmood, U. C11 Methionine PET (MET-PET) Imaging of Glioblastoma for Detecting Postoperative Residual Disease and Response to Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1024–1028.
- Zhou, W.; Zhou, Z.; Wen, J.; Xie, F.; Zhu, Y.; Zhang, Z.; Xiao, J.; Chen, Y.; Li, M.; Guan, Y.; et al. A Nomogram Modeling (11)C-MET PET/CT and Clinical Features in Glioma Helps Predict IDH Mutation. Front. Oncol. 2020, 10, 1200.
- Park, Y.J.; Lee, J.W.; Cho, H.W.; Choe, Y.S.; Lee, K.H.; Choi, J.Y.; Sung, K.W.; Moon, S.H. Value of C-11 methionine PET/CT in patients with intracranial germinoma. PLoS ONE 2022, 17, e0263690.
- Morales-Lozano, M.I.; Viering, O.; Samnick, S.; Rodriguez-Otero, P.; Buck, A.K.; Marcos-Jubilar, M.; Rasche, L.; Prieto, E.; Kortüm, K.M.; San-Miguel, J.; et al. (18)F-FDG and (11)C-Methionine PET/CT in Newly Diagnosed Multiple Myeloma Patients: Comparison of Volume-Based PET Biomarkers. Cancers 2020, 12, 1042.
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564.
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Tran, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 6539–6544.
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 2021, 131, e140100.
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763.
- Dunphy, M.P.S.; Harding, J.J.; Venneti, S.; Zhang, H.; Burnazi, E.M.; Bromberg, J.; Omuro, A.M.; Hsieh, J.J.; Mellinghoff, I.K.; Staton, K.; et al. In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of (18)F-(2S,4R)-4-Fluoroglutamine. Radiology 2018, 287, 667–675.
- Cohen, A.S.; Grudzinski, J.; Smith, G.T.; Peterson, T.E.; Whisenant, J.G.; Hickman, T.L.; Ciombor, K.K.; Cardin, D.; Eng, C.; Goff, L.W.; et al. First-in-Human PET Imaging and Estimated Radiation Dosimetry of l--Glutamine in Patients with Metastatic Colorectal Cancer. J. Nucl. Med. 2022, 63, 36–43.
- Venneti, S.; Dunphy, M.P.; Zhang, H.; Pitter, K.L.; Zanzonico, P.; Campos, C.; Carlin, S.D.; La Rocca, G.; Lyashchenko, S.; Ploessl, K.; et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci. Transl. Med. 2015, 7, 274ra217.
- Baek, S.; Mueller, A.; Lim, Y.S.; Lee, H.C.; Lee, Y.J.; Gong, G.; Kim, J.S.; Ryu, J.S.; Oh, S.J.; Lee, S.J.; et al. (4S)-4-(3-18F-fluoropropyl)-L-glutamate for imaging of xC transporter activity in hepatocellular carcinoma using PET: Preclinical and exploratory clinical studies. J. Nucl. Med. 2013, 54, 117–123.
- Yelamanchi, S.D.; Jayaram, S.; Thomas, J.K.; Gundimeda, S.; Khan, A.A.; Singhal, A.; Keshava Prasad, T.S.; Pandey, A.; Somani, B.L.; Gowda, H. A pathway map of glutamate metabolism. J. Cell Commun. Signal 2016, 10, 69–75.
- Zhu, L.; Ploessl, K.; Zhou, R.; Mankoff, D.; Kung, H.F. Metabolic Imaging of Glutamine in Cancer. J. Nucl. Med. 2017, 58, 533–537.
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684.
- Herman, S.; Niemelä, V.; Emami Khoonsari, P.; Sundblom, J.; Burman, J.; Landtblom, A.M.; Spjuth, O.; Nyholm, D.; Kultima, K. Alterations in the tyrosine and phenylalanine pathways revealed by biochemical profiling in cerebrospinal fluid of Huntington’s disease subjects. Sci. Rep. 2019, 9, 4129.
- Baumann, U.; Duhme, V.; Auth, M.K.; McKiernan, P.J.; Holme, E. Lectin-reactive alpha-fetoprotein in patients with tyrosinemia type I and hepatocellular carcinoma. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 77–82.
- Nguyen, T.N.; Nguyen, H.Q.; Le, D.H. Unveiling prognostics biomarkers of tyrosine metabolism reprogramming in liver cancer by cross-platform gene expression analyses. PLoS ONE 2020, 15, e0229276.
- Sun, L.; Zhang, L.; Chen, J.; Li, C.; Sun, H.; Wang, J.; Xiao, H. Activation of Tyrosine Metabolism in CD13+ Cancer Stem Cells Drives Relapse in Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 604–621.
- Lohmann, P.; Werner, J.M.; Shah, N.J.; Fink, G.R.; Langen, K.J.; Galldiks, N. Combined Amino Acid Positron Emission Tomography and Advanced Magnetic Resonance Imaging in Glioma Patients. Cancers 2019, 11, 153.
- Spaeth, N.; Wyss, M.T.; Weber, B.; Scheidegger, S.; Lutz, A.; Verwey, J.; Radovanovic, I.; Pahnke, J.; Wild, D.; Westera, G.; et al. Uptake of 18F-fluorocholine, 18F-fluoroethyl-L-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: Implications for separation of radiation necrosis from tumor recurrence. J. Nucl. Med. 2004, 45, 1931–1938.
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.W.; Zilles, K.; Coenen, H.H.; Langen, K.J. O-(2-fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687.
- Baguet, T.; Verhoeven, J.; De Vos, F.; Goethals, I. Cost-Effectiveness of Fluoroethyl-L-Tyrosine for Temozolomide Therapy Assessment in Patients With Glioblastoma. Front. Oncol. 2019, 9, 814.
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J.O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214.
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015, 17, 1293–1300.
- Cavanagh, B.L.; Walker, T.; Norazit, A.; Meedeniya, A.C. Thymidine analogues for tracking DNA synthesis. Molecules 2011, 16, 7980–7993.
- Chen, G.; Deng, X. Cell Synchronization by Double Thymidine Block. Bio Protoc. 2018, 8, e2994.
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338.
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351.
- Poon, M.A.; O’Connell, M.J.; Wieand, H.S.; Krook, J.E.; Gerstner, J.B.; Tschetter, L.K.; Levitt, R.; Kardinal, C.G.; Mailliard, J.A. Biochemical modulation of fluorouracil with leucovorin: Confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J. Clin. Oncol. 1991, 9, 1967–1972.
- von Forstner, C.; Egberts, J.H.; Ammerpohl, O.; Niedzielska, D.; Buchert, R.; Mikecz, P.; Schumacher, U.; Peldschus, K.; Adam, G.; Pilarsky, C.; et al. Gene expression patterns and tumor uptake of 18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse xenotransplantation model of pancreatic cancer. J. Nucl. Med. 2008, 49, 1362–1370.
- McKinley, E.T.; Ayers, G.D.; Smith, R.A.; Saleh, S.A.; Zhao, P.; Washington, M.K.; Coffey, R.J.; Manning, H.C. Limits of -FLT PET as a biomarker of proliferation in oncology. PLoS ONE 2013, 8, e58938.
- Cieslak, J.A.; Sibenaller, Z.A.; Walsh, S.A.; Ponto, L.L.; Du, J.; Sunderland, J.J.; Cullen, J.J. Fluorine-18-Labeled Thymidine Positron Emission Tomography (FLT-PET) as an Index of Cell Proliferation after Pharmacological Ascorbate-Based Therapy. Radiat. Res. 2016, 185, 31–38.
- Collet, S.; Valable, S.; Constans, J.M.; Lechapt-Zalcman, E.; Roussel, S.; Delcroix, N.; Abbas, A.; Ibazizene, M.; Bernaudin, M.; Barré, L.; et al. -fluoro-L-thymidine PET and advanced MRI for preoperative grading of gliomas. Neuroimage Clin. 2015, 8, 448–454.
- Chen, X.; Yang, Y.; Katz, S.I. Dexamethasone pretreatment impairs the thymidylate synthase inhibition mediated flare in thymidine salvage pathway activity in non-small cell lung cancer. PLoS ONE 2018, 13, e0202384.
- Aravind, P.; Popat, S.; Barwick, T.D.; Soneji, N.; Lythgoe, M.; Lozano-kuehne, J.; Sreter, K.B.; Bergqvist, M.; Patel, N.H.; Aboagye, E.O.; et al. Fluorothymidine(FLT)-PET imaging of thymidine kinase 1 pharmacodynamics in non-small cell lung cancer treated with pemetrexed. J. Clin. Oncol. 2022, 40, 3070.
- Volante, M.; Righi, L.; Berruti, A.; Rindi, G.; Papotti, M. The pathological diagnosis of neuroendocrine tumors: Common questions and tentative answers. Virchows. Arch. 2011, 458, 393–402.
- Jager, P.L.; Chirakal, R.; Marriott, C.J.; Brouwers, A.H.; Koopmans, K.P.; Gulenchyn, K.Y. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: Basic aspects and emerging clinical applications. J. Nucl. Med. 2008, 49, 573–586.
- Santhanam, P.; Taïeb, D. Role of (18) F-FDOPA PET/CT imaging in endocrinology. Clin. Endocrinol. 2014, 81, 789–798.
- Stormezand, G.N.; Chaves, L.T.; Vállez García, D.; Doorduin, J.; De Jong, B.M.; Leenders, K.L.; Kremer, B.P.H.; Dierckx, R. Intrastriatal gradient analyses of 18F-FDOPA PET scans for differentiation of Parkinsonian disorders. Neuroimage Clin. 2020, 25, 102161.
- Treglia, G.; Cocciolillo, F.; de Waure, C.; Di Nardo, F.; Gualano, M.R.; Castaldi, P.; Rufini, V.; Giordano, A. Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1144–1153.
- Sun, R.C.; Young, L.E.A.; Bruntz, R.C.; Markussen, K.H.; Zhou, Z.; Conroy, L.R.; Hawkinson, T.R.; Clarke, H.A.; Stanback, A.E.; Macedo, J.K.A.; et al. Brain glycogen serves as a critical glucosamine cache required for protein glycosylation. Cell Metab. 2021, 33, 1404–1417.e1409.
- Allott, L.; Brickute, D.; Chen, C.; Braga, M.; Barnes, C.; Wang, N.; Aboagye, E.O. Development of a fluorine-18 radiolabelled fluorescent chalcone: Evaluated for detecting glycogen. EJNMMI Radiopharm. Chem. 2020, 5, 17.
- Witney, T.H.; Carroll, L.; Alam, I.S.; Chandrashekran, A.; Nguyen, Q.D.; Sala, R.; Harris, R.; DeBerardinis, R.J.; Agarwal, R.; Aboagye, E.O. A novel radiotracer to image glycogen metabolism in tumors by positron emission tomography. Cancer Res. 2014, 74, 1319–1328.
