Xylan is the most abundant hemicellulose, constitutes about 25–35% of the dry biomass of woody and lignified tissues, and occurs up to 50% in some cereal grains. The accurate degree and position of xylan acetylation is necessary for xylan function and for plant growth and development. The post synthetic acetylation of cell wall xylan, mainly regulated by Reduced Wall Acetylation (RWA), Trichome Birefringence-Like (TBL), and Altered Xyloglucan 9 (AXY9) genes, is essential for effective bonding of xylan with cellulose. Recent studies have proven that not only xylan acetylation but also its deacetylation is vital for various plant functions.
- xylan
- cell wall
- acetylation
- deacetylation
- biosynthesis
- esterases
1. Introduction
Xylan is the most abundant type of hemicellulose that occurs abundantly in cell walls of land plants, where it accounts for more than 30% of the dry weight, while in primary walls, it accounts for about 20% and its composition depends on the origin [1]. There is a lot of diversity in xylan structures as it depends upon the source of its origin. Generally, xylan is a heteropolymer with a backbone made of a β-(1→4)-D-xylospyranose backbone bearing 4-O-methyl-α-D-glucopyranosyl acid and α-L-arabinosyl and other monosaccharide side chains [2]. Depending upon the side chain on the xylan backbone, they can be divided into three major classes: glucuronoxylan, glucuronoarabinoxylan, and arabinoxylan. Glucuronoxylans are abundant in secondary walls of dicots and some non-grass monocots [2,3], glucuronoarabinoxylans are abundant in grasses and gymnosperms except members from Gnetophyta [4,5], and arabinoxylans are abundant in cereal grains [6,7]. In dicots and some non-grass monocots, glucuronoxylan made up to 25% of total weight of secondary walls. The glucuronoarabinoxylan is present in gymnosperm softwood [1] and grass species [2].
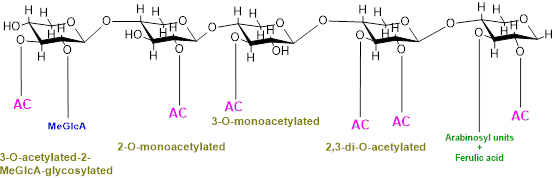
In addition, backbone may also be substituted with O-linked methyl, acetyl, and feruloyl groups which protect polysaccharides from specific glycosyl hydrolases and cross-link cell-wall constituents controlling cell extensibility [8,9]. O-Acetylation is a common and prevalent method of xylan modification and is a ubiquitous substitution within hemicellulose families [10–12]. Cell wall polysaccharides are either mono- or di- acetylated as revealed by a study on ten out of fourteen cell walls constituting polysaccharides substituted with acetyl groups [10]. Furthermore, the positions of acetylation of these cell polysaccharides also vary, for example, xylose in xylan is acetylated at the O-2 and/or O-3 positions, galactose and/or mannose are acetylated at the O-6 position, and mannose in mannan/glucomannan is acetylated at the O-2 and/or O-3 positions [13,14]; fucose at O-6 and O-4 and galactose at the O-3 position in xyloglucan are also acetylated [15]. Acetylation of xylan can be of four different types, i.e., xylospyranose residues may be acetylated at the 2-O position, and thus, xylan will be called 2-O-monoacetylated; xylospyranose residues may be acetylated at the O-3 position and thus 3-O-monoacetylated; and xylospyranose residues may be acetylated at both the O-2 and O-3 positions and thus regarded as 2,3-di-O-acetylated. Finally, a xylospyranose residue may contain an acetyl group at the 3-O position and MeGlcA substitution at position O-3 is called 3-O-acetylated-2-MeGlcA-glycosylated xylan. In grasses, arabinose is attached at position three while the acetyl group is attached at position O-2 of xylopyronose residues [16] (Figure 1).
Figure 1. Types of xylan acetylation in woody plants and grasses.
Although the exact degree of acetylation of cell-wall polymers is not yet known, many studies reveal that acetylation varies with plant type, tissue type, developmental stages, and cell wall [17–22]. For example, xylospyranose backbone of hardwood xylan is 70% acetylated at the C-2 and/or C-3 positions while softwood xylospyranose usually lack acetylation [11]. In poplar, as demonstrated by a recent study, the acetate can reach about 6.7% (w/w) of wood biomass [23]. Similarly, different plants or plant organs differ considerably in the types and content of xylan substitution, e.g., in the Populus trichocarpa stem, 63% of the total xylan is acetylated, of which 23.6% xylan possesses acetyl substitution at the O-2 position while 15.8% possesses acetyl substitution at the O-3 position, 14.8% xylan was substituted at both the O-2 and 3 positions, and 9.1% xylan has 3Ac-2GlcA substitution [24].
2. Difference in Substitution Patterns of Xylan
Glucuronoxylan has xylospyranose residues in its backbone connected via 1,4-linkages and contains acetyl and glucuronic acid or its derivatives as backbone substitutions and is common in many dicots [25]. In methylglucuronoxylan, the xylospyranose backbone is substituted with 4-O-methylgluconic acid at the O-2 position and has a common occurrence in birch and eucalyptus [19,26]. The specific positions of acetyl and methylgluconic acid on the xylan backbone have recently been demonstrated in study by [27]. Arabinoglucuronoxylan and glucuronoarabinoxylan are common in the arabinosyl and methylgluconic acid groups at the O-3 and O-2 positions, respectively. Both are different in the content of these two-sided chains along with the acetyl content. For example, non-acetylated arabinoglucuronoxylan from spruce has a higher O-4 methylgluconic acid substitution than arabinose [28,29]. The specific pattern of arabinose and methyl gluconic acid substitution in arabinoglucuronoxylan has recently been established [30]. Glucuronoarabinoxylan from sugarcane straw and bagasse xylans have either single or double substitution of arabinose with a lower methylgluconic acid content, while it is highly acetylated in hardwood and softwood [31]. The difference in the degree of xylan acetylation affects the physical and chemical properties of xylan, e.g., acetylation significantly affects the solubility as well as the water content of glucuronoxylans in aspen wood with small effect on molecular weight [32]. Furthermore, xylan acetylation enhances the thermal tolerance, mechanical strength, and hydrophobicity ideal for industrial utilization of xylan [32,33].
3. Substrate for Xylan Acetylation
Being the hub of all metabolic pathways, acetyl CoA regulates the metabolism of almost all essential nutrients and molecules needed to sustain life, including sugars, fats, and proteins [34–38]. It can be synthesized through multiple processes in the cell including glycolysis, the phosphoketolase pathway, and the Wood–Ljungdahl pathway in multiple cell organelles including plastid, mitochondrion, cytosol, and peroxisome [39–43]. Cytosolic acetyl CoA is a source of acetyl group for the acetylation of various types of metabolites, such as alkaloids, anthocyanins, isoprenoids, and phenols with a variety of commercial applications [44]. Although it was initially not clear which acetyl CoA pool is the exact source of xylan acetylation, recent studies have confirmed that cytosolic acetyl CoA is the sole donor of the xylan acetylation acetyl group [45]. A heteromeric enzyme ATP-citrate lyase (ACL) consisting of ACLA and ACLB subunits is responsible for synthesis of cytosolic acetyl-CoA as downregulation of antisense RNA of ACLA-1 in Arabidopsis leads to abnormal plant growth and reduced accumulation of multiple acetyl-CoA derivatives, e.g., stem cuticular wax and flavonoids in seeds [46]. Many In vitro studies have confirmed that polysaccharide esterases, i.e., XOATs, MOATs, and XGOATs associated with cell walls, can use acetyl-CoA as a substrate to transfer acetyl groups onto their respective oligosaccharide acceptors but that there was no evidence to support this argument in living plants until 2018. There are two problems with acetyl CoA acting as a donor of acetyl groups for xylan or other hemicellulose acetylation; firstly, there is no known acetyl-CoA-generating pathway in Golgi, and secondly, the lipid membrane is impermeable to acetyl-CoA [44]. Due to the impermeability of the Golgi membrane to acetyl CoA, there must be an intermediate that could facilitate the transport of acetyl CoA across the membranes. Experimental evidence for this confusion was provided from a study conducted by [45], that identified multi-transmembrane RWA proteins (RWA1, RWA2, RWA3, and RWA4) as facilitators for the transfer of acetyl CoA to the Golgi. In plants, RWA proteins mainly consist of two clades AB and CD, RWAs belonging to CD clade transport acetyl CoA to the Golgi for xylan acetylation in Populus [47]. The presence of multi-transmembrane proteins or domains in bacterial or plants polysaccharide O-acetylating systems involved in transmembrane transport of acetyl CoA and self-acetylating across membranes is not known yet [48].
4. Mechanism of Xylan Acetylation
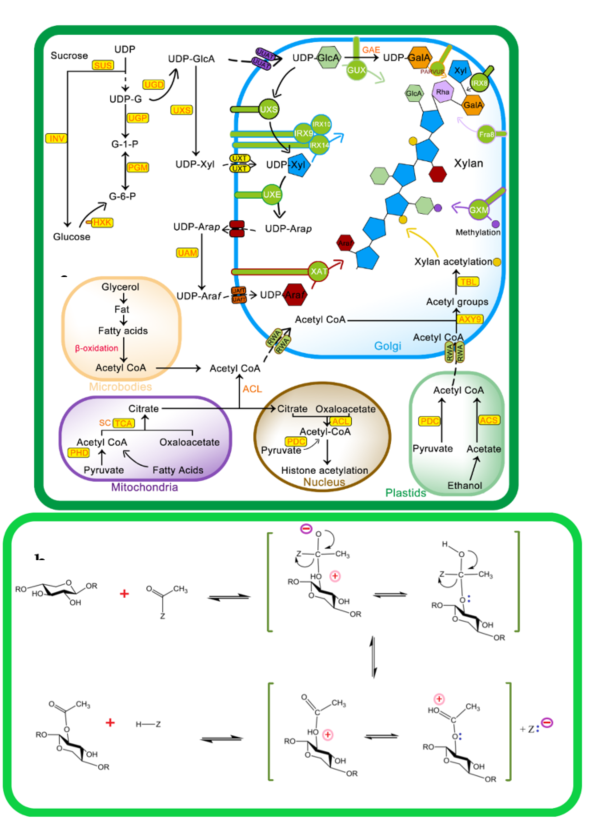
Recent studies have shown that three main groups of proteins, i.e., RWA (Reduced Wall Acetylation), Trichome Birefringence-Like (TBL), and AXY9 (Altered Xyloglucan 9), are involved in cell-wall polymer O-acetylation (Figure 2 a,b). The polysaccharide O-acetyltransferases from the TBL family are well known for their ability to acetyl cell-wall polymers [49–51]. The enzymes encoded by the TBL family share the TBL and DUF231 domains with the Arabidopsis Trichome Birefringence protein and contain the Gly-Asp-Ser and the Asp-x-x-His conserve motifs [10,52,53]. Till now, a number of members of the TBL gene family being studied for their role in regiospecific acetylation of xylan backbone, e.g., from Arabidopsis TBL35 (XOAT9), TBL34 (XOAT8), TBL31 (XOAT5), TBL32 (XOAT6), TBL33 (XOAT7), TBL30 (XOAT3), TBL28 (XOAT2), TBL3 (XOAT4), and recently TBL10 is identified [20,49–51,54]. The structure and mechanistic details of XOAT1 is published recently; it not only catalyzes acetylation of xylosyl residue at O–2 position but also facilitates nonenzymatic transfer of acetyl group to the O-3 position. The mechanism of XOAT1 mediated acetylation involves a double displacement bi-bi mechanism involving a Ser-His-Asp catalytic triad which results in formation of the acyl-enzyme intermediate and uses an Arginine (Arg) residue for oxyanion hole formation. An important factor during transitional state of mechanism catalyzed by the Ser-His-Asp triad is oxyanion hole formation that regulate extra negative charge on acetyl group oxygen [55]. The Agr (Arg-219) residue in the conserved RNQxxS motif of TBL-block II is present in the active site and stabilizes negative charge during tetrahedral reaction intermediate formation [55].
RWA family proteins are considered an important component of wall polysaccharide acetylation as they are involved in the transfer of acetyl CoA from the cytoplasm to Golgi. RWAs contain multiple transmembrane helices similar to the transmembrane regions of the CAS1 fungal protein glucuronoxylomannan acetylation [18]. Four members of the RWA family, i.e., RWA1, RWA2, RWA3, and RWA4 have been reported in Arabidopsis, and any mutation in these causes a significant decrease in wall acetylation [56,57]. The Role of AXY9 intermediate acetyl donor substrate has recently been proposed, with GDS and DxxH patterns homologous to the TBL family [58] and weak acetyl esterase activity [24]. AXY9 may therefore act as an acetyl donor for xylan acetylation from other sources, i.e., pseudo-substrates, 4-methylumbelliferyl acetate, and p-nitrophenyl acetate [24], other than acetyl CoA and may form an acyl-AXY9 intermediate, which may either act as a protein-activated acetyl donor or as an intermediate step in the formation of an unknown acetyl donor, but further research needs to confirm this [56].
As shown in Figure 2, initially, RWA proteins facilitate transport of cytosolic or acetyl CoA synthesized in other subcellular organelles to the Golgi [57,59]. Recent evidence suggests that RWA protein acetylates the proposed intermediate, i.e., AXY9 [58], and subsequently, TBL29 transfers the acetyl group to the xylan backbone [60,61]. The degree of xylan acetylation is regulated by a Golgi-localized BS1 (BRITTLE LEAF SHEATH1) protein as the BS1 mutant lacks specific xylan acetylation patterns [62]. The search for BS1 orthologues in plants is on its way to understand occurrence and mechanisms of xylan deacetylation in the Golgi [63].
Figure 2. Mechanism of xylan biosynthesis and acetylation: (A) sucrose synthesized from photosynthesis is major source for UDP-glucose (UDP-G), which serves as a substrate for synthesis of various intermediates involved in xylan side chain or backbone synthesis. The Golgi is the actual site for xylan synthesis, so all substrates are transported to the Golgi via different membrane transporters. Acetyl CoA, a donor of acetyl group for xylan acetylation, is synthesized in different cell compartments i.e., microbodies, mitochondria, and plastids as well as in the cytosol from where it is transported to Golgi via Reduced Wall Acetylation (RWA) proteins and later incorporated to xylan. (b) Molecular mechanism of xylan acetylation adapted from [64,65]: attachment of acetyl to xylan involves nucleophilic attack of xylan OH group lone pair electrons on carbon atoms of the acetyl group to yield acetylated xylan. Abbreviations of all enzymes and intermediates are mentioned in Abbreviation section of manuscript
This entry is adapted from the peer-reviewed paper 10.3390/ijms21217875