Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Blue carbon was established as a metaphor to highlight that, apart from terrestrial ecosystems, coastal ecosystems also contribute significantly to carbon sequestration. Apart from being recognized as a helpful carbon sink, blue carbon ecosystems provide various other services, including shelter for different migratory birds, fishes, and crabs. It is also vital in minimizing net carbon emissions.
- blue carbon
- climate change
- biofuels
1. Spatiotemporal Distribution of Blue Carbon Ecosystems
Coastal vegetated habitats including salt marshes, seagrasses, and mangroves have long provided humans with advantages. More recently, notwithstanding data limitations, their importance as carbon reserves has been recognized in climate change mitigation [1][2]. As illustrated in Figure 1, spatiotemporal distribution and the importance of blue carbon ecosystems in controlling climate change can be seen [3].
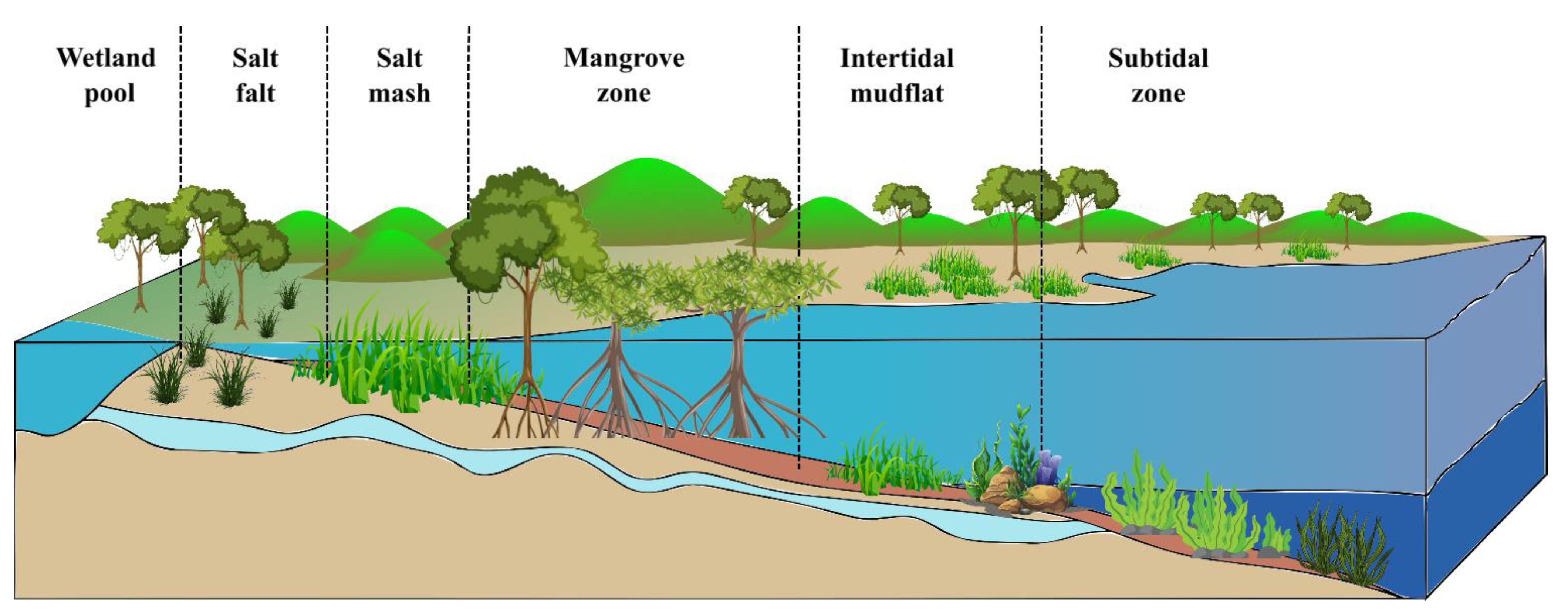
Figure 1. Spatiotemporal distribution and the importance of blue carbon ecosystems.
Coastal zone environments, including seagrass beds, rocky reefs and corals, intertidal marshes, sandy beaches, kelp forests, and mangrove forests [4], help combat climate change by effectively storing and sequestering CO2, known as “coastal Blue Carbon” [5]. Salt marshes, seagrasses, and mangroves, for example, often form a spatially connected continuum of intertidal ecosystems. Unvegetated mudflats and sandbars are ecosystems that contain and sequester vast quantities of organic carbon [6][7]. Blue carbon soil is anaerobic, mainly in contrast to the terrestrial ground, which causes carbon stored in these soils to decay at a slow rate, and thus the carbon accumulates for hundreds to thousands of years [8][9]. The coastal wetland vegetation acts as a buffer zone between land and oceans, capable of storing surplus water during the rainy season and preventing floods [10]. They also help to protect coastlines and are considered to be more cost-effective than complicated structures such as seawalls and levees, as they are cheaper to manage and will be able to keep up with rising sea levels [11][12]. They also exhibit high burial rates leading to the seafloor’s rise, acting as a barrier against rising sea levels and wave actions linked with climate change [13]. They serve as a motivator for ecosystem-based adaptation to protect humans, infrastructure, and property from the negative impacts of climate change [14].
Mangroves occur in tropical and sub-tropical regions [15]. They are found in 118 countries worldwide, with 15 countries accounting for 75% of their overall coverage. West Africa is home to nearly a quarter of the world’s mangroves, containing almost 0.854 billion metric tons of carbon in below-ground and above-ground biomass [16]. Similarly, Indonesia alone accounts for 23% [17]. But a considerable loss of 0.16–0.39% per year has been recorded in the mangroves since 2000 [18]. Mangroves have an excellent ability to store carbon in the root system and act as carbon-rich forests in the tropics; hence their management and conservation need to be prioritized [19]. Geological evidence indicates the adaptation of mangroves to earlier climate and sea-level change [20][21]. They play a crucial role in promoting sedimentation in sensitive coastal regions, hence withstanding climate-induced impacts such as rising sea levels [22]. Their wide variety of aerial root structures such as pneumatophores, prop roots, plank roots, and knee roots help prevent soil erosion and differ in their efficacy to reserve sediments [23]. Moreover, mangroves speed up land development through a rise in sedimentation, lower wave exposure, and peat formation, consequently mitigating exposure to tropical storm surges and sea-level rise [24].
Seagrasses are another blue carbon ecosystem found mainly in shallow coastal margins across zero latitudes. Seagrasses use photosynthesis to take in carbon dioxide and assimilate it into their biomass. The above-ground/water vegetation traps suspended particulate matter (sedimentation) that later adds to the sedimentary storage component [25]. They have colossal mitigation potential for neutralizing CO2 emissions, which leads to improvements in carbon estimates stored in seagrass sediments and incorporate seagrass ecosystems [26][27]. The total global area under seagrass ranges from 300,000–600,000 km2 [28]. However, there has been a sharp decrease in recent decades, with a sevenfold decline reported from 1990 to 2009 [29]. Globally, seagrasses are declining by 2–5% each year as 30,000 Km2 of seagrass have been destroyed in recent decades [30]. Every year, organic carbon oxidation in degraded seagrass meadows potentially releases 0.03–0.33 petagrams of carbon dioxide back into the atmosphere [31]. Seagrasses cover 4.8 million hectares in West Africa, holding an estimated 673 million tons of carbon [16]. In coastal waters, the restoration of seagrasses has led to increased sequestration of blue carbon [32]. However, they have a poor carbon storage capacity compared to mangroves.
Unlike seagrasses and mangroves, salt marshes differ in having low methane emissions [33][34]. Salt marshes cover 1.2 million hectares in West Africa, holding 303 million metric tons of CO2 [16]. In recent studies, an area of 45,000 Km2 has been reported for salt marshes [35]. Apart from carbon sinks, they are prodigious inorganic carbon sources of coastal oceans [36]. Tidal marshes, mapped only in 43 countries of the world, represent 14% of the global coastal area [37]. The minimum yearly global loss rate of tidal marshes is 1–2% [38]. Although the blue carbon ecosystems have proved their ability as ideal carbon sinks, both natural and artificial threats destroy these ecosystems. Due to the rise in sea level, marshes sink to stress and shrink with time [39]. Further, marine accidents, such as massive oil spills, are also responsible for the damage to these ecosystems [40][41][42]. Hence, to avail the maximum benefits of these ecosystems, proper policymaking and guiding mechanisms should be established to preserve and manage these blue carbon ecosystems.
Other coastal ecosystems such as barrier islands, dunes, and beaches made of sand, play a pivotal role in dispersing wave energy, besides having vital sediment reserves that aid in preserving coastlines, and to a certain extent, in adapting to rising sea levels [43][44]. It is debatable if coral reefs are the sinks or sources of atmospheric CO2 [45]. However, they make remarkable structures, ranging from deep oceans to their surfaces and parallel to coastlines in many places extending up to several kilometres, in such a way that they form a significant part of the coastal defense. The mass flow of energy from overlying waters into the coral systems significantly reduces wave activity—a vital function of reef roughness [46][47]. However, as per Pendleton et al. [31], enormous reserves of carbon sequestered in the past are affected by the transformation of these coastal ecosystems, as blue carbon present in the sediments is released into the atmosphere when these ecosystems are degraded [31]. As a result, the value of blue carbon habitats in sequestering organic carbon has boosted conservation efforts as a means to reduce climate change and offset CO2 emissions [48]. Furthermore, their contribution to strengthening coastal resilience to weather disasters and changing climate has led to their participation in many countries’ nationwide defined commitments (NDCs) for climate change adaptation and mitigation [49][50].
2. Blue Carbon as a Potential Source for Biofuel Production
The sustainability of the first-generation bio-based fuels (1G) was also called into doubt since their utilization threatened the traditional food supply, particularly in developing nations [51][52]. The second-generation biofuels (2G/cellulosic biofuels) which were derived from cellulosic energy crops including municipal solid wastes, lignocellulosic residues, or agro-industrial wastes, provided an alternative option because of their plentiful availability [53][54][55][56][57][58]. However, this type of fuel also coped with a lot of failures due to higher investment expenses and technical problems in down streaming. Furthermore, the generation of 1G/2G biofuels necessitated additional crop cultivation acreage, and hence they could not be viewed as a viable alternative to fossil fuels because the yield gained might not fulfil the global energy demand. In addition, the third-generation biofuels (3G/advanced biofuels) were made from aquatic biomass such as algae [59][60]. Algae gained a lot of interest among third-generation biofuels because of their low lignin concentration and high productivity, which reduced the consumption of energy throughout fuel generation [61][62]. Moreover, blue carbon sources were biomasses that were morphologically and systematically more similar to plants on the ground than seaweeds [63]. Hence, exploiting blue carbon sources appeared to be an appealing solution for renewable energy generation, avoiding the significant drawbacks related to 1G and 2G bio-derived fuels. Indeed, the biofuel production pathway from blue carbon sources could be illustrated in Figure 2.
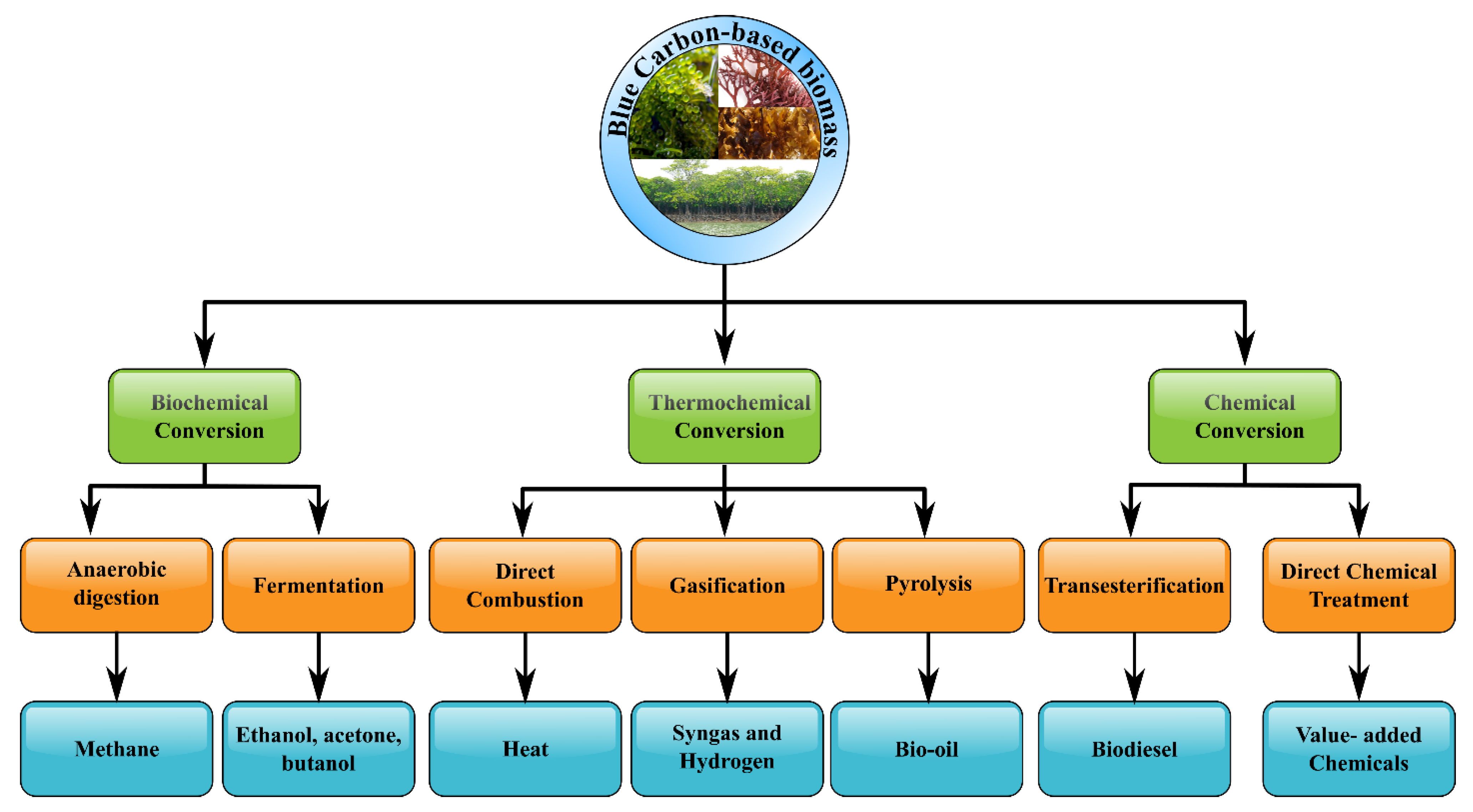
Figure 2. Suggested technologies for biofuel production from blue carbon sources-based biomass.
It could be seen from Figure 2 that transesterification, direct combustion, gasification, or pyrolysis were all methods for producing biofuel from dry blue carbon-based biomass [64][65][66][67]. Meanwhile, energy generation techniques from wet blue carbon-based biomass included enzyme hydrolysis, hydrothermal treatments, anaerobic digestion, and fermentation to biobutanol/bioethanol/biohydrogen [68][69][70][71]. It was noted that utilizing blue carbon-based biomass for biofuel generation was in the early stages of research and development. Besides, a lot of non-glucose-derived sugars such as cell wall polysaccharides and mannitol were accumulated in seaweed, but not so many glucose-originated polysaccharides [72]. As a result, industrial bioethanol synthesis from blue carbon-based biomass necessitated the fermentation of not only non-glucose but also glucose-based sugars [73]. For chemical compositions, the blue carbon sources and terrestrial plants differed substantially in general. For example, seaweeds have high water content (90% fresh wt), protein content (from 7 to 15% dry wt), carbohydrate content (25–50% dry wt), as well as low concentration of lipid (between 1 and 5% dry wt) in comparison with terrestrial biomass [74].
As reported, the lipid content in the blue carbon sources was low; however, their carbohydrate content was high, permitting them to be employed as a feedstock for the generation of different fermentative bio-base fuels [75]. Though fermentation facilities using macroalgae were known as relatively expensive to operate and build, they were dependable and provided large yields [76]. This is partly because of the high content of water (from 70 to 90%), the protein concentration of around 10%, and the presence of various amounts of carbohydrates [77]. Furthermore, because there was a small amount of lignin and hemicellulose in macroalgae cells, the enzymatic and chemical pretreatment stages in the production of biofuel were removed [78]. More significantly, the carbohydrate concentration of macroalgae varied greatly depending on strains, species, and cultivars. In addition, because the potential growth speed and carbohydrate concentration of the green macroalgae Ulva lactuca were high, it was thought of as a promising aquatic energy crop [79]. Regarding brown macroalgae Laminaria spp., there could be up to 55% carbohydrates in it with dry weight, principally free sugars, cellulose, hemicellulose as well as the energy storage molecules mannitol and laminarin [80]. Indeed, biohydrogen generation from blue carbon received a lot of interest because of carbohydrate-rich blue carbon. In a study by Yukesh et al. [81], they improved the generation of biohydrogen from seagrass using new ozone-linked rotor-stator homogenization. In particular, rotor-stator homogenization required 510 kJ/kg TS of specific energy to accomplish 10.45% seagrass lysis while ozone-linked rotor-stator homogenization obtained 23.7% seagrass lysis with less energy (only 212.4 kJ/kg TS) input. It was noted that the ozone-coupled rotor-stator homogenization sample’s biohydrogen generation capability was evaluated and compared with using biohydrogenesis.
The generation of biogas was considered a long-time technology. Interestingly, there were multiple operational biogas systems, ranging from large-scale to small-scale and they were supplied by a variety of feedstocks such as animal wastes, agricultural products, certain residential rubbish, and sewage sludge [82][83]. Additionally, because macroalgae contained more water than terrestrial biomass (ranging between 80 and 85%), they were more suited for microbial conversion instead of thermochemical conversion [84][85]. Indeed, producing biogas from macroalgae was more technically feasible than generating biogas from other fuels because all organic components in macroalgae (such as protein, carbohydrates, and so on) could be transformed into biogas via anaerobic digestion [86][87]. Besides, a low lignocellulose concentration of macroalgae facilitated biodegradation more than that of their relative microalgae to create considerable amounts of biogas [88][89]. However, microalgae could be cultivated using pollutant water and CO2 [90][91] and could be used to synthesize many types of biofuels such as bioethanol, biodiesel, and bio-oil [92][93][94][95].
Many works successfully established the practical usefulness of seaweed as a feedstock for the anaerobic digestion process. For example, the generation of biogas from marine wrack might reduce GHG emissions while also bringing economic benefits to local island people. Apart from that, Marquez et al. [96] discovered biogas generation by employing three different microbial seeds including marine sediment, marine wrack-related microflora, and manure of cow. Accordingly, the authors discovered that the average biogas generated was 1223 mL from marine wrack-related microflora, 2172 mL from marine sediment, and 551 mL from the manure of cow. Although the methane potential at 396.9 mL CH4/g volatile solid was calculated using marine wrack proximate values in comparison with other feedstock, this parameter was low when the greatest methane yield of 94.33 mL CH4/g volatile solid was considered. Interestingly, among the microbial seeds tested, sediment in the marine platform was found to be the most effective source of microorganisms in terms of using seawater and marine wrack biomass to produce biogas. Nonetheless, sand deposition in salinity and digesters might cause trouble in the long-term anaerobic digestion process [97][98]. As observed, several factors, including growing method, species type, harvesting time, and seaweed production per hectare all made a great contribution to the anaerobic digestion process. It was noted that the balance of material and energy, harvesting biomass cost, carbon balance, as well as expenses of creating biogas from seaweed were not evaluated [99][100]. As reported, methane yields in biogas produced from the anaerobic digestion process of blue carbon sources could be changed with biochemical composition and they were linked to ash concentration and the degree of sugars stored [88]. Therefore, to increase methane yields, Banu et al. [101] used disperser-tenside (polysorbate 80) disintegration so as to improve the biomethanation ability of seagrass (namely Syringodium isoetifolium). Indeed, dispersion-assisted tenside disintegration had a more significant influence on bio-acidification as well as biomethanation assays in terms of methane production (0.256 g/g COD) and volatile fatty acid content (1100 mg/L) when compared to dispersion disintegration, which was 0.198 g/g COD; 800 mg/L. As a result, S. isoetifolium was seen as a potential substrate for achieving third-generation biofuel targets in the foreseeable future.
Apart from that, marine algae, which contained a high concentration of hydrolyzable carbohydrates, cellulose, glucan, and galactan, might serve as a possible feedstock to produce liquid biofuels [102]. As reported, two popular liquid transportation biofuels are synthetic biodiesel, bioethanol, and biobutanol using marine macroalgae feedstock. In comparison with edible as well as lignocellulosic biomass sources, marine macroalgae biomass was gaining popularity as a renewable feedstock to produce bioethanol [88][103]. As mentioned above, macroalgae possessed a high carbohydrate concentration and low lignin [104], making them appropriate for use as a substrate in the fermentation process to generate bioethanol after hydrolysis. The current techniques for bioethanol synthesis from seaweed were separate hydrolysis and fermentation, and simultaneous saccharification and fermentation, as illustrated in Figure 3 [74][105][106]. As for separate hydrolysis and fermentation, seaweed biomass was hydrolyzed before being fermented in discrete units using yeast or bacteria [72][107]. Regarding simultaneous saccharification and fermentation, however, both fermentation and hydrolysis occurred concurrently in a single stage [108][109].
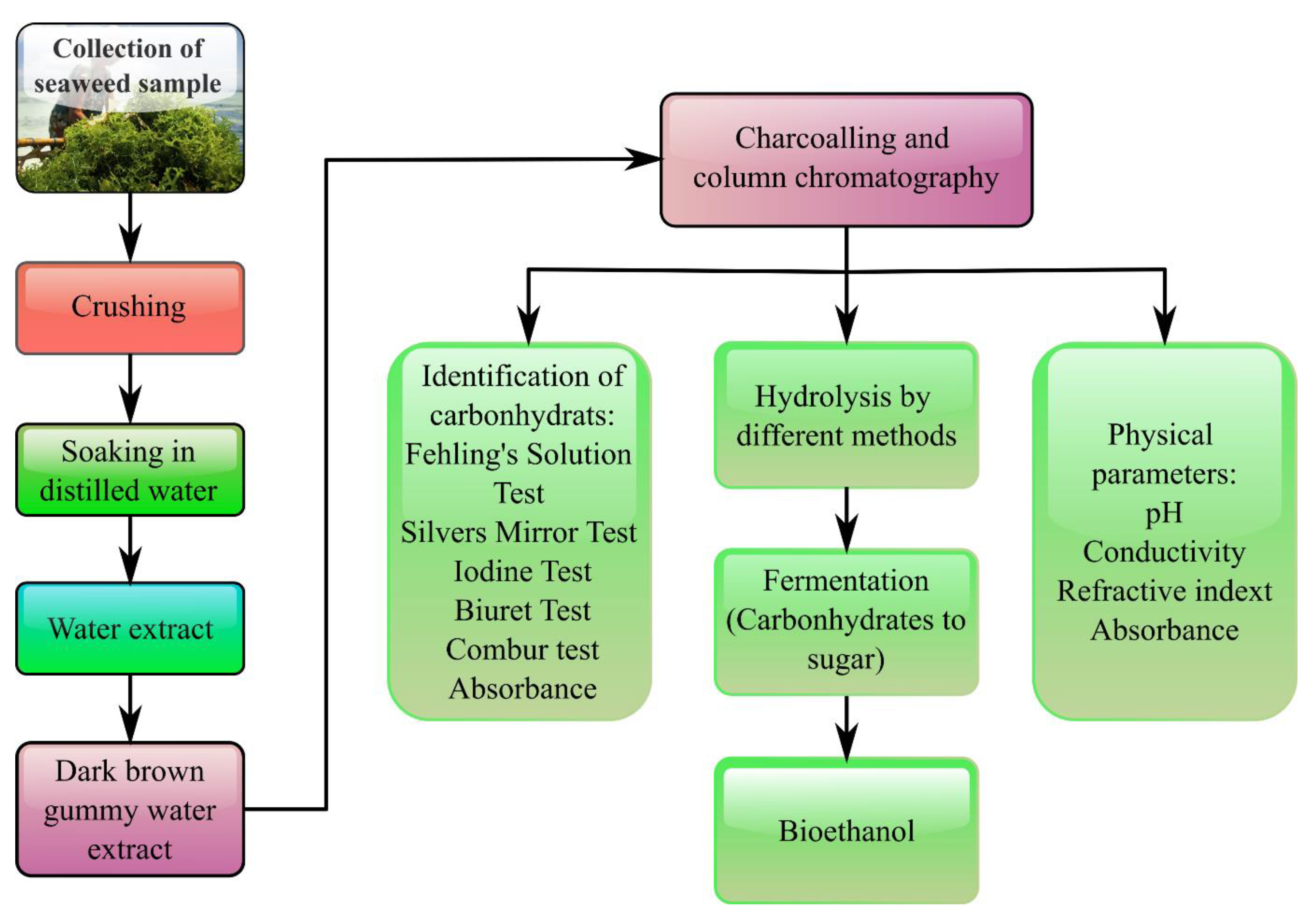
Figure 3. Scheme of bioethanol production from seaweed via hydrolysis and fermentation process.
Even though experiments on bioethanol generation from macroalgae were scarce, it was not hard to determine that using marine macroalgae waste for bio-derived fuel feedstock could lead to less rivalry for biofuels among food [75][110]. According to several investigations, the findings of using seagrass biowaste for bioethanol production appeared to be promising in terms of making this a reality [111][112][113]. In an investigation by Mahmoud et al. [114], they employed seven samples of beach-cast seagrasses (associated with Z. marina, S. filiforme, Z. noltii, P. australis, T. testudinum, and P. oceanic) gathered from maritime environments worldwide with carbohydrate concentration ranging between 73% and 81% (w/dry weight of biomass). With no pretreatment, enzymatic hydrolysis with a single step was designed to effectively extract the monomeric sugars present in biomass originating from seagrass. In shake flasks, P. oceanica hydrolysate was observed to produce higher lipid yields (at 6.8 g/L) in comparison with the synthetic minimum medium (just 5.1 g /L). Additionally, it was then used as the only fermentation medium for oleaginous yeast T. oleaginous under the technical scale with the use of a fed-batch bioreactor, yielding 224.5 g /L lipids (0.35 g /L.h). Furthermore, the proportion of sugar/lipid conversion (w/w) was seen to be 0.41. According to cumulative statistics, roughly 4 million tons of microbial oils might be created by harvesting just half of the beach-cast seagrass in the world. Besides, Ravikumar et al. [111] presented their research on manufacturing bioethanol from seagrass biowastes with the use of Saccharomyces cerevisiae. The greatest bioethanol generation (0.047 mL/g) was observed in fresh seagrass leaves under acid pretreatment. As a result, fresh seagrass leaves might be one of the appropriate substrates for bioethanol synthesis. Furthermore, an investigation by Uchida et al. [115] studied the seagrass seeds (Zostera marina) bioethanol fermentation. On a dry weight basis, there were 83.5% carbs in the seeds, which included 48.1% crude starch. This parameter was equivalent to that of cereals such as corn and wheat flour. As reported, the saccharification of seeds went smoothly with no heating pretreatment, which showed that the starch present in seagrass seeds possessed a molecular form being ready to be digested by glucoamylase. Besides, the authors proposed that it might be possible to develop alcoholic drinks and foods from seagrass seeds, resulting in the creation of a unique marine fermentation sector in the future. The treatment of Laminaria japonica, Gelidium amansii, Ulva fasciata, Ulva lactuca, and Sargassum fulvellum biomass with acid and hydrolytic enzymes resulted in hydrolyzates with distinct proportions of mannose, glucose, mannitol, galactose, and other sugars [116]. As reported, Laminaria japonica hydrolyzate produced 0.4 g bioethanol for each gram of carbohydrate in case hydrolytic enzymes were utilized [117]. In another study, Adams et al. [118] investigated the generation of ethanol through laminarin polysaccharide yeast fermentation from the brown macroalga Saccharina latissimi using a variety of pretreatments. Meanwhile, in an experiment by Wi et al. [102], fermentation pretreatments were researched for a red microalgae species (namely Ceylon moss) with a high carbohydrate concentration (normally 23% galactose and 20% glucose). Accordingly, they proved that pretreatment approaches could be utilized to broaden the range of macroalga species appropriate for bioethanol generation. Moreover, Ge et al. [119] investigated the utilization of floating residual wastes from the industry of alginate from Laminaria japonica (a brown alga) to generate bioethanol after they were pretreated with diluted sulphuric acid as well as experienced enzymatic hydrolysis. Likewise, Horn et al. [120] showed the ability of fermented extracts of Laminaria hyporbea to synthesize ethanol with the employment of Pichia angophorea (yeast), while El-Sayed et al. [121] assessed the utilization of reducing sugars from U. lactuca to produce bioethanol via Saccharomyces cerevisiae.
In the case of biobutanol, there existed just a few studies that researched the manufacture of biobutanol from macroalgae. In other words, macroalgae, especially brown algae, and their potential for biochemical transformation to butanol and other solvents by Clostridium spp. via acetone-butanol fermentation were not studied. However, the brown macroalgae biomass’s acetone butanol fermentation feasibility via C. acetobutylicum was proved, and the results showed that the butanol content in the hydrolysate reached around 0.26 g butanol/g sugar while 0.29 g butanol/g sugar was obtained in the pilot investigation [122][123]. In addition, HMF was regarded as among the chemical platforms that have the most potential for the conversion of industrially important bio-originated chemical compounds. According to several researchers, a greater starch concentration was accumulated in seagrass seeds [124][125]. Moreover, several studies showed that raw biomass sources rich in non-structural carbohydrates, such as sucrose, fructose, starch, and glucose were employed as biomaterials for HMF generation [126][127]. Furthermore, by utilizing beach-cast seagrasses without feedstock expenses, seagrass feedstocks might contribute to sustainably and cost-effectively manufacturing HMF, which showed that seagrass biomasses were considered the most attractive source of bio-based feedstock to produce HMF sustainably.
Macroalgae were used to produce biogas and bioethanol instead of biodiesel since they lacked triglycerides. Typically, macroalgae were transformed into bio-derived oils such as free fatty acids and lipids, and more importantly, the lipids were separated to generate bio-based diesel. Even though free fatty acids were a precursor to biodiesel, the excessive quantity of free fatty acids in the oil might stymie the intended transformation. In an experiment, Tamilarasan [128] esterified the free fatty acids in Enteromorpha compressa algal oil from 6.3% to 0.34%, and subsequently, two stages for biodiesel synthesis were developed. More notably, Xu [129] recently tried to use macroalgae as a carbon source for oleaginous yeast aiming to create bio-based diesel, and the maximal lipid concentration was observed to reach 48.30%. In contrast, the by-product-free fatty acids accompanying mannitol could be utilized to cultivate the oleaginous yeast. Also, several innovative approaches, such as ultrasonic irradiation, were employed to support transesterification through the formation of fine emulsions between alcohol and oil, and the rate of reaction was enhanced due to cavitation [128]. Furthermore, biodiesel output from wet biomass achieved was nearly 10 times lower compared to that obtained from dry biomass, suggesting that water had a detrimental influence on transesterification experiments, and hence the dehydration process was required to attain high efficiency [130]. Moreover, Saengsawang et al. [131] investigated whether Rhizoclonium sp. oil could be employed as a biodiesel alternative to optimize the reaction conditions required for the process of chemical transesterification. The biodiesel weight of 0.174 ± 0.034 g along with 82.2% of the whole FAME was produced during the transesterification procedure from macroalgae oil. Besides, this research indicated that biodiesel produced from Rhizoclonium might be utilized as an alternative fuel, and more research would make it appropriate for large-scale manufacturing.
Thermochemical techniques are also considered potential solutions for converting biomass sources into biofuels [132][133][134]. Indeed, pyrolysis was the most used technique for extracting bio-oil [84] [135]. Pyrolytic cracking could quickly transform dried seaweed biomass into bio-originated oil and solid residue. Furthermore, investigations on the behaviors of pyrolysis and product properties of some macroalgae, such as brown algae, green algae, and red algae [92][136], revealed that the macroalgae’s pyrolysis process to produce biofuels and that of terrestrial plants were alike [137][138], even though the macroalgae had higher activation energy than that of terrestrial biomass [139]. Importantly, pyrolysis of macroalgae operating under 500 °C was shown to be a favorable temperature for maximizing bio-oil output [108][140]. Liquefaction was seen as a process where biomass experienced complex thermochemical reactions in a solvent solution, resulting in mostly liquid products. Remarkably, hydrothermal liquefaction mostly neglected macroalgae in the role of a feedstock for bio-originated oil since microalgae were assumed to have a greater lipid concentration intrinsically [141][142]. Elliott et al. [143] reported on the hydrothermal liquefaction of Macrocystis sp. with the employment of a batch reactor that was fed with 10% kelp dry mass in water. According to the oil product’s solvent separation, an oil yield of 19.2 wt% was observed. Utilizing Na2CO3 as a catalyst, Zhou et al. [144] investigated the hydrothermal liquefaction of the green marine macroalgae named Enteromorpha prolifera and got a maximal bio-oil output of 23.0% dw as well as an energy density of 29.89 MJ/kg. In another study, Neveux et al. [145] used hydrothermal liquefaction in a batch reactor to convert six types of freshwater and marine green macroalgae into bio-crude. The findings showed that the high ash concentration of macroalgae caused poorer bio-oil yields when compared to the results achieved from hydrothermal liquefaction of a variety of microalgae (in the range of 26–57% dw) [146]. Although the gasification of biomass on a wide scale was successfully demonstrated, it was still comparatively costly in contrast to fossil-fuel energy [147][148]. Indeed, gasification was able to generate hydrogen and syngas at a competitive price in the market. Actually, several nations had very few pilot gasification factories, more widespread industrial penetration appeared to be dependent on integration into the chain of biofuel from seaweed [149]. Table 1 compared and showed the benefits and drawbacks of several biofuel generation methods from blue carbon sources.
| Processing Techniques | Target Products | Benefits | Drawbacks |
|---|---|---|---|
| Anaerobic digestion | Biogas | Finishing technology without drying process | High inhibition and salt |
| Fermentation | Bioethanol/biobutanol | High content of carbohydrate | Low efficiency in forming various mixed sugars |
| Transesterification | Biodiesel | No required the dewatering process | Low yield |
| Pyrolysis/Gasification/Liquefaction | Bio-oil, syngas, hydrogen, bio-char | Fast rate without required chemicals | High energy consumption |
This entry is adapted from the peer-reviewed paper 10.3390/su15032682
References
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdrés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon. A Rapid Response Assessment; United Nations Environment Programme, GRID-Arendal: Nairobi, Kenya, 2009.
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193.
- OzCoasts. About Conceptual Diagrams 2022. Available online: https://ozcoasts.org.au/conceptual-diagrams/introduction/ (accessed on 18 March 2022).
- Ruttenberg, B.; Granek, E. Bridging the marine–terrestrial disconnect to improve marine coastal zone science and management. Mar. Ecol. Prog. Ser. 2011, 434, 203–212.
- Wylie, L.; Sutton-Grier, A.E.; Moore, A. Keys to successful blue carbon projects: Lessons learned from global case studies. Mar. Policy 2016, 65, 76–84.
- Phang, V.X.H.; Chou, L.M.; Friess, D.A. Ecosystem carbon stocks across a tropical intertidal habitat mosaic of mangrove forest, seagrass meadow, mudflat and sandbar. Earth Surf. Process Landf. 2015, 40, 1387–1400.
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175.
- Hiraishi, T.; Krug, T.; Tanabe, K.; Srivastava, N.; Baasansuren, J.; Fukuda, M. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Geneva, Switzerland, 2014.
- Herr, D.; Landis, E. Coastal blue carbon ecosystems. Opportunities for Nationally Determined Contributions. Policy Brief 2016, 1–28.
- Zhou, C.; Wong, K.; Zhao, J. Coastal Wetland Vegetation in Response to Global Warming and Climate Change. In Sea Level Rise and Coastal Infrastructure; InTech: London, UK, 2018.
- Beck, M.W.; Lange, G.M. Guidelines for Coastal and Marine Ecosystem Accounting: Incorporating the Protective Service Values of Coral Reefs and Mangroves in National Wealth Accounts, Wealth Accounting and Valuation of Ecosystem Services; World Bank: Washington, DC, USA, 2015; pp. 1–15.
- Narayan, S.; Beck, M.W.; Reguero, B.G.; Losada, I.J.; van Wesenbeeck, B.; Pontee, N. The Effectiveness, Costs and Coastal Protection Benefits of Natural and Nature-Based Defences. PLoS ONE 2016, 11, e0154735.
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968.
- Narayan, S.; Beck, M.W.; Wilson, P.; Thomas, C.; Guerrero, A.; Shepard, C. Coastal Wetlands and Flood Damage Reduction. Using Risk Industry-Based Models to Assess Natural Defenses in the Northeastern USA; Loyd’s Tercentenary Research Foundation: London, UK, 2012; pp. 1–23.
- Alongi, D. The Energetics of Mangrove Forests, 1st ed.; Springer: Dordrecht, The Netherlands, 2009.
- Bryan, T.; Virdin, J.; Vegh, T.; Kot, C.Y.; Cleary, J.; Halpin, P.N. Blue carbon conservation in West Africa: A first assessment of feasibility. J. Coast. Conserv. 2020, 24, 8.
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159.
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738.
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297.
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39.
- Woodroffe, C.D.; Rogers, K.; McKee, K.L.; Lovelock, C.E.; Mendelssohn, I.A.; Saintilan, N. Mangrove Sedimentation and Response to Relative Sea-Level Rise. Ann. Rev. Mar. Sci. 2016, 8, 243–266.
- Hoque, M.M.; Abu Hena, M.K.; Ahmed, O.H.; Idris, M.H.; Hoque, A.T.M.R.; Billah, M.M. Can mangroves help combat sea level rise through sediment accretion and accumulation? Malaysian J. Sci. 2015, 34, 78–86.
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34.
- Chow, J. Mangrove management for climate change adaptation and sustainable development in coastal zones. J. Sustain. For. 2018, 37, 139–156.
- Ramesh, R.; Banerjee, K.; Paneerselvam, A.; Raghuraman, R.; Purvaja, R.; Lakshmi, A. Importance of Seagrass Management for Effective Mitigation of Climate Change. Coast. Manag. 2019, 283–299.
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310.
- Ricart, A.M.; York, P.H.; Bryant, C.V.; Rasheed, M.A.; Ierodiaconou, D.; Macreadie, P.I. High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci. Rep. 2020, 10, 5865.
- Charpy-Roubaud, C.; Sournia, A. The comparative estimation of phytoplanktonic, microphytobenthic and macrophytobenthic primary production in the oceans. Mar. Microb. Food Webs 1990, 4, 31–57.
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381.
- Sundararaju, V. Why We Must Conserve the World’s Seagrasses; Wildlife Biodiversity: New Delhi, India, 2020.
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e43542.
- Greiner, J.T.; McGlathery, K.J.; Gunnell, J.; McKee, B.A. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE 2013, 8, e72469.
- Drake, K.; Halifax, H.; Adamowicz, S.C.; Craft, C. Carbon sequestration in tidal salt marshes of the Northeast United States. Environ. Manag. 2015, 56, 998–1008.
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity influence on methane emissions from tidal marshes. Wetlands 2011, 31, 831–842.
- Greenberg, R.; Maldonado, J.E.; Droege, S.A.M.; McDonald, M.V. Tidal marshes: A global perspective on the evolution and conservation of their terrestrial vertebrates. Bioscience 2006, 56, 675–685.
- Wang, Z.A.; Kroeger, K.D.; Ganju, N.K.; Gonneea, M.E.; Chu, S.N. Intertidal salt marshes as an important source of inorganic carbon to the coastal ocean. Limnol. Oceanogr. 2016, 61, 1916–1931.
- Mcowen, C.J.; Weatherdon, L.V.; Van Bochove, J.-W.; Sullivan, E.; Blyth, S.; Zockler, C. A global map of saltmarshes. Biodivers. Data J. 2017, 5, e11764.
- Duarte, C.M.; Dennison, W.C.; Orth, R.J.W.; Carruthers, T.J.B. The Charisma of Coastal Ecosystems: Addressing the Imbalance. Estuaries Coasts 2008, 31, 233–238.
- Doody, J.P. ‘Coastal squeeze’—An historical perspective. J. Coast. Conserv. 2004, 10, 129–138.
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910.
- Wang, H.; Liu, Z.; Wang, X.; Graham, T.; Wang, J. An analysis of factors affecting the severity of marine accidents. Reliab. Eng. Syst. Saf. 2021, 210, 107513.
- Ghasemi, O.; Mehrdadi, N.; Baghdadi, M.; Aminzadeh, B.; Ghaseminejad, A. Spilled oil absorption from Caspian sea water by graphene/chitosan nano composite. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 2856–2872.
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12.
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Meliane, I.; Hale, L.Z.; Shepard, C.C. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manag. 2014, 90, 50–57.
- Chisholm, J.R.M.; Barnes, D.J. Anomalies in coral reef community metabolism and their potential importance in the reef CO2 source-sink debate. Proc. Natl. Acad. Sci. USA 1998, 95, 6566–6569.
- Kench, P.S.; Brander, R.W. Wave Processes on Coral Reef Flats: Implications for Reef Geomorphology Using Australian Case Studies. J. Coast. Res. 2006, 221, 209–223.
- Monismith, S.G. Hydrodynamics of Coral Reefs. Annu. Rev. Fluid Mech. 2007, 39, 37–55.
- Geraldi, N.R.; Ortega, A.; Serrano, O.; Macreadie, P.I.; Lovelock, C.E.; Krause-Jensen, D. Fingerprinting Blue Carbon: Rationale and Tools to Determine the Source of Organic Carbon in Marine Depositional Environments. Front. Mar. Sci. 2019, 6, 263.
- Herr, D.; von Unger, M.; Laffoley, D.; McGivern, A. Pathways for implementation of blue carbon initiatives. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 116–129.
- Lovelock, C.E.; Reef, R. Variable Impacts of Climate Change on Blue Carbon. One Earth 2020, 3, 195–211.
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Al-Muhtaseb, A.H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845.
- Manikandan, S.; Subbaiya, R.; Biruntha, M.; Krishnan, R.Y.; Muthusamy, G.; Karmegam, N. Recent development patterns, utilization and prospective of biofuel production: Emerging nanotechnological intervention for environmental sustainability—A review. Fuel 2022, 314, 122757.
- Son Le, H.; Said, Z.; Tuan Pham, M.; Hieu Le, T.; Veza, I.; Nhanh Nguyen, V. Production of HMF and DMF biofuel from carbohydrates through catalytic pathways as a sustainable strategy for the future energy sector. Fuel 2022, 324, 124474.
- Karimi-Maleh, H.; Rajendran, S.; Vasseghian, Y.; Dragoi, E.-N. Advanced integrated nanocatalytic routes for converting biomass to biofuels: A comprehensive review. Fuel 2022, 314, 122762.
- Bamisaye, A.; Ige, A.R.; Adegoke, I.A.; Ogunbiyi, E.O.; Bamidele, M.O.; Adeleke, O. Eco-friendly de-lignified and raw Celosia argentea waste solid biofuel: Comparative studies and machine learning modelling. Fuel 2023, 340, 127412.
- Maaoui, A.; Ben Hassen Trabelsi, A.; Hamdi, M.; Chagtmi, R.; Jamaaoui, F.; Lopez, G. Towards local circular economy through Opuntia Ficus Indica cladodes conversion into renewable biofuels and biochars: Product distribution and kinetic modelling. Fuel 2023, 332, 126056.
- Tuan Hoang, A.; Nižetić, S.; Ölçer, A.I.; Chyuan Ong, H. Synthesis pathway and combustion mechanism of a sustainable biofuel 2,5-Dimethylfuran: Progress and prospective. Fuel 2021, 286, 119337.
- Bin, Y.; Yu, Z.; Huang, Z.; Li, M.; Zhang, Y.; Ma, X. Investigation on the co-pyrolysis of municipal solid waste and sawdust: Pyrolysis behaviors, kinetics, and thermodynamic analysis. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 8001–8011.
- Kowthaman, C.N.; Senthil Kumar, P.; Arul Mozhi Selvan, V.; Ganesh, D. A comprehensive insight from microalgae production process to characterization of biofuel for the sustainable energy. Fuel 2022, 310, 122320.
- Wu, Y.; Xu, X.; Jiang, X.; Lin, J.; Lin, X.; Zhao, S. Valorisation of harmful algae bloom (Enteromorpha prolifera) for polysaccharide and crude bio-oil production. Fuel 2022, 324, 124482.
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Kandasamy, S.; Ramasamy Subramanian, K.P. A review on hydrothermal liquefaction of algal biomass on process parameters, purification and applications. Fuel 2022, 313, 122679.
- Liu, J.; Zhou, F.; Abed, A.M.; Le, B.N.; Dai, L.; Elhosiny Ali, H. Macroalgae as a potential source of biomass for generation of biofuel: Artificial intelligence, challenges, and future insights towards a sustainable environment. Fuel 2023, 336, 126826.
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996.
- Mumtaz, M.; Baqar, Z.; Hussain, N.; Afifa Bilal, M.; Azam, H.M.H. Application of nanomaterials for enhanced production of biodiesel, biooil, biogas, bioethanol, and biohydrogen via lignocellulosic biomass transformation. Fuel 2022, 315, 122840.
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.-H. Biomass torrefaction: An overview of process and technology assessment based on global readiness level. Fuel 2022, 324, 124663.
- Wang, P.; Xu, P.; Wang, B.; Shen, C.; Shen, L. Green ammonia production via microalgae steam catalytic gasification process over LaFeO3 perovskite. Fuel 2022, 318, 123322.
- Xu, D.; Lin, J.; Ma, R.; Hou, J.; Sun, S.; Ma, N. Fast pyrolysis of algae model compounds for bio-oil: In-depth insights into the volatile interaction mechanisms based on DFT calculations. Fuel 2023, 333, 126449.
- Agrawal, R.; Bhadana, B.; Singh Chauhan, P.; Adsul, M.; Kumar, R.; Gupta, R.P. Understanding the effects of low enzyme dosage and high solid loading on the enzyme inhibition and strategies to improve hydrolysis yields of pilot scale pretreated rice straw. Fuel 2022, 327, 125114.
- Yuan, C.; Zhao, S.; Ni, J.; He, Y.; Cao, B.; Hu, Y. Integrated route of fast hydrothermal liquefaction of microalgae and sludge by recycling the waste aqueous phase for microalgal growth. Fuel 2023, 334, 126488.
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M. Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel 2022, 324, 124554.
- Germec, M.; Karhan, M.; Demirci, A.; Turhan, I. Kinetic modeling, sensitivity analysis, and techno-economic feasibility of ethanol fermentation from non-sterile carob extract-based media in Saccharomyces cerevisiae biofilm reactor under a repeated-batch fermentation process. Fuel 2022, 324, 124729.
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel production in Ireland—An approach to 2020 targets with a focus on algal biomass. Energies 2013, 6, 6391–6412.
- Mushlihah, S.; Langford, A.; Tassakka, A.C.M.A.R. Ozonolysis as an effective pretreatment strategy for bioethanol production from marine algae. BioEnergy Res. 2020, 13, 1269–1279.
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew Sustain. Energy Rev. 2018, 91, 165–179.
- El-Sheekh, M.M.; Ibrahim, H.A.H.; Barakat, K.M.; Shaltout, N.A.; Sayed, W.M.M.E.L.; Abou-Shanab, R.A.I. Potential of Marine Biota and Bio-Waste Materials as Feedstock for Biofuel Production; Waste Management; CRC Press: Boca Raton, FL, USA, 2022; pp. 123–139.
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784.
- Quiroz-Arita, C.; Shinde, S.; Kim, S.; Monroe, E.; George, A.; Quinn, J.C. Bioproducts from high-protein algal biomass: An economic and environmental sustainability review and risk analysis. Sustain. Energy Fuels 2022, 6, 2398–2422.
- Özçimen, D.; Inan, B. An Overview of Bioethanol Production From Algae. In Biofuels—Status and Perspective; InTech: London, UK, 2015.
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour Technol. 2011, 102, 2595–2604.
- Chelf, P.; Brown, L.M.; Wyman, C.E. Aquatic biomass resources and carbon dioxide trapping. Biomass Bioenergy 1993, 4, 175–183.
- Kannah, R.Y.; Kavitha, S.; Gunasekaran, M.; Kumar, G.; Banu, J.R.; Zhen, G. Biohydrogen production from seagrass via novel energetically efficient ozone coupled rotor stator homogenization. Int. J. Hydrogen Energy 2020, 45, 5881–5889.
- Demirbas, A. Use of algae as biofuel sources. Energy Convers Manag. 2010, 51, 2738–2749.
- Alnaqi, A.A.; Alsarraf, J.; Al-Rashed, A.A.A.A. The waste heat of a biofuel-powered SOFC for green hydrogen production using thermochemical cycle; Economic, environmental analysis, and tri-criteria optimization. Fuel 2023, 335, 126599.
- Fakayode, O.A.; Wahia, H.; Zhang, L.; Zhou, C.; Ma, H. State-of-the-art co-pyrolysis of lignocellulosic and macroalgae biomass feedstocks for improved bio-oil production—A review. Fuel 2023, 332, 126071.
- Yameen, M.Z.; AlMohamadi, H.; Naqvi, S.R.; Noor, T.; Chen, W.-H.; Amin, N.A.S. Advances in production & activation of marine macroalgae-derived biochar catalyst for sustainable biodiesel production. Fuel 2023, 337, 127215.
- Yaashikaa, P.R.; Keerthana Devi, M.; Senthil Kumar, P. Algal biofuels: Technological perspective on cultivation, fuel extraction and engineering genetic pathway for enhancing productivity. Fuel 2022, 320, 123814.
- Nanda, M.; Jaiswal, K.K.; Negi, J.; de Farias Neves, F.; Ranjitha, J.; Vlaskin, M.S. A sustainable approach to produce yeast lipid by utilizing marine macroalgae biomass. Fuel 2023, 338, 127214.
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew Sustain. Energy Rev. 2015, 47, 427–437.
- Andrade, C.; Martins, P.L.; Duarte, L.C.; Oliveira, A.C.; Carvalheiro, F. Development of an innovative macroalgae biorefinery: Oligosaccharides as pivotal compounds. Fuel 2022, 320, 123780.
- Kim, S.; Kim, M.; Chang, Y.K.; Kim, D. Lipid production under a nutrient-sufficient condition outperforms starvation conditions due to a natural polarization of lipid content in algal biofilm. Fuel 2022, 126902.
- Peter, A.P.; Chew, K.W.; Pandey, A.; Lau, S.Y.; Rajendran, S.; Ting, H.Y. Artificial intelligence model for monitoring biomass growth in semi-batch Chlorella vulgaris cultivation. Fuel 2023, 333, 126438.
- Sathya, A.B.; Thirunavukkarasu, A.; Nithya, R.; Nandan, A.; Sakthishobana, K.; Kola, A.K. Microalgal biofuel production: Potential challenges and prospective research. Fuel 2023, 332, 126199.
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543.
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782.
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Vasseghian, Y. Engineering strategies and opportunities of next generation biofuel from microalgae: A perspective review on the potential bioenergy feedstock. Fuel 2022, 312, 122827.
- Marquez, G.P.B.; Reichardt, W.T.; Azanza, R.V.; Klocke, M.; Montaño, M.N.E. Thalassic biogas production from sea wrack biomass using different microbial seeds: Cow manure, marine sediment and sea wrack-associated microflora. Bioresour Technol. 2013, 133, 612–617.
- Pechsiri, J.S.; Thomas, J.-B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M. Energy performance and greenhouse gas emissions of kelp cultivation for biogas and fertilizer recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355.
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation potential of macroalgae Ulva spp. and Gracilaria spp. and in co-digestion with waste activated sludge. Bioresour Technol. 2012, 114, 320–326.
- Peu, P.; Sassi, J.-F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F. Sulphur fate and anaerobic biodegradation potential during co-digestion of seaweed biomass (Ulva sp.) with pig slurry. Bioresour Technol. 2011, 102, 10794–10802.
- Thakur, N.; Salama, E.-S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient utilization and management of seaweed biomass for biogas production. Mater Today Sustain. 2022, 18, 100120.
- Banu, J.R.; Tamilarasan, K.; Rani, R.U.; Gunasekaran, M.; Cho, S.-K.; Ala’a, H. Dispersion aided tenside disintegration of seagrass Syringodium isoetifolium: Towards biomethanation, kinetics, energy exploration and evaluation. Bioresour Technol. 2019, 277, 62–67.
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol. 2009, 100, 6658–6660.
- Alam, S.N.; Khalid, Z.; Guldhe, A.; Singh, B.; Korstad, J. Harvesting and pretreatment techniques of aquatic macrophytes and macroalgae for production of biofuels. Environ. Sustain. 2021, 4, 299–316.
- Abomohra, A.E.-F.; El-Naggar, A.H.; Baeshen, A.A. Potential of macroalgae for biodiesel production: Screening and evaluation studies. J. Biosci Bioeng 2018, 125, 231–237.
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew Sustain. Energy Rev. 2016, 65, 756–769.
- Bibi, R.; Ahmad, Z.; Imran, M.; Hussain, S.; Ditta, A.; Mahmood, S. Algal bioethanol production technology: A trend towards sustainable development. Renew Sustain. Energy Rev. 2017, 71, 976–985.
- Maceiras, R.; Rodrı, M.; Cancela, A.; Urréjola, S.; Sánchez, A. Macroalgae: Raw material for biodiesel production. Appl. Energy 2011, 88, 3318–3323.
- Li, R.; Zhong, Z.; Jin, B.; Zheng, A. Selection of temperature for bio-oil production from pyrolysis of algae from lake blooms. Energy Fuels 2012, 26, 2996–3002.
- Ye Lee, J.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125.
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Pandian, P.L. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125362.
- Ravikumar, S.; Gokulakrishnan, R.; Kanagavel, M.; Thajuddin, N. Production of biofuel ethanol from pretreated seagrass by using Saccharomyces cerevisiae. Indian J. Sci. Technol. 2011, 4, 1087–1089.
- Viola, E.; Cardinale, M.; Santarcangelo, R.; Villone, A.; Zimbardi, F. Ethanol from eel grass via steam explosion and enzymatic hydrolysis. Biomass Bioenergy 2008, 32, 613–618.
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078.
- Masri, M.A.; Younes, S.; Haack, M.; Qoura, F.; Mehlmer, N.; Brück, T. A Seagrass-Based Biorefinery for Generation of Single-Cell Oils for Biofuel and Oleochemical Production. Energy Technol. 2018, 6, 1026–1038.
- Uchida, M.; Miyoshi, T.; Kaneniwa, M.; Ishihara, K.; Nakashimada, Y.; Urano, N. Production of 16.5% v/v ethanol from seagrass seeds. J. Biosci Bioeng 2014, 118, 646–650.
- Ghazal, M.A.; Ibrahim, H.A.H.; Shaltout, N.A.; Ali, A.E. Biodiesel and bioethanol production from Ulva fasciata Delie biomass via enzymatic pretreatment using marine-derived Aspergillus niger. Int. J. Pure App. Biosci. 2016, 4, 1–6.
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437.
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J. Appl. Phycol. 2009, 21, 569–574.
- Ge, L.; Wang, P.; Mou, H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew. Energy 2011, 36, 84–89.
- Horn, S.J.; Aasen, I.M.; Østgaard, K. Production of ethanol from mannitol by Zymobacter palmae. J. Ind. Microbiol. Biotechnol. 2000, 24, 51–57.
- El-Sayed, W.M.M.; Ibrahim, H.A.H.; Abdul-Raouf, U.M.; El-Nagar, M.M. Evaluation of bioethanol production from Ulva lactuca by Saccharomyces cerevisiae. J. Biotechnol. Biomater. 2016, 6, 2.
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309.
- Potts, T.; Du, J.; Paul, M.; May, P.; Beitle, R.; Hestekin, J. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sustain. Energy 2012, 31, 29–36.
- De Rosa, S.; Zavodnik, N.; De Stefano, S.; Fiaccavento, R.; Travizi, A. Seasonal Changes of Biomass and Soluble Carbohydrates in the Seagrass Zostera noltii Hornem; Walter de Gruyter: Berlin, Germany, 1990.
- Syed, F.; Zakaria, M.H.; Bujang, J.S.; Ramaiya, S.D.; Hayashizaki, K. Physicochemical properties of starches from seed and rhizome of Enhalus acoroides. Phil J. Nat. Sci. 2019, 24, 27–33.
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422.
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Toward sustainable hydroxymethylfurfural production using seaweeds. Trends Biotechnol. 2020, 38, 487–496.
- Tamilarasan, S.; Sahadevan, R. Ultrasonic assisted acid base transesterification of algal oil from marine macroalgae Caulerpa peltata: Optimization and characterization studies. Fuel 2014, 128, 347–355.
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461.
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381.
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841.
- Khan, M.; Raza Naqvi, S.; Ullah, Z.; Ali Ammar Taqvi, S.; Nouman Aslam Khan, M.; Farooq, W. Applications of machine learning in thermochemical conversion of biomass-A review. Fuel 2023, 332, 126055.
- Siwal, S.S.; Sheoran, K.; Saini, A.K.; Vo, D.-V.N.; Wang, Q.; Thakur, V.K. Advanced thermochemical conversion technologies used for energy generation: Advancement and prospects. Fuel 2022, 321, 124107.
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Jaiswal, K.K.; Kumar, V.; Kumar, S. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351.
- Xu, K.; Li, J.; Zeng, K.; Zhong, D.; Peng, J.; Qiu, Y. The characteristics and evolution of nitrogen in bio-oil from microalgae pyrolysis in molten salt. Fuel 2023, 331, 125903.
- Li, D.; Chen, L.; Chen, S.; Zhang, X.; Chen, F.; Ye, N. Comparative evaluation of the pyrolytic and kinetic characteristics of a macroalga (Sargassum thunbergii) and a freshwater plant (Potamogeton crispus). Fuel 2012, 96, 185–191.
- Wang, S.; Wang, Q.; Jiang, X.; Han, X.; Ji, H. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Convers. Manag. 2013, 68, 273–280.
- Kan, T.; Grierson, S.; De Nys, R.; Strezov, V. Comparative assessment of the thermochemical conversion of freshwater and marine micro-and macroalgae. Energy Fuels 2014, 28, 104–114.
- Ngoc Bao Dung, T.; Lay, C.-H.; Nguyen, D.D.; Chang, S.W.; Rajesh Banu, J.; Hong, Y. Improving the biohydrogen production potential of macroalgal biomass through mild acid dispersion pretreatment. Fuel 2023, 332, 125895.
- Bae, Y.J.; Ryu, C.; Jeon, J.-K.; Park, J.; Suh, D.J.; Suh, Y.-W. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520.
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377.
- Moazezi, M.R.; Bayat, H.; Tavakoli, O.; Hallajisani, A. Hydrothermal liquefaction of Chlorella vulgaris and catalytic upgrading of product: Effect of process parameter on bio-oil yield and thermodynamics modeling. Fuel 2022, 318, 123595.
- Elliott, D.C.; Sealock, L.J., Jr.; Butner, R.S. Product analysis from direct liquefaction of several high-moisture biomass feedstocks. In Pyrolysis Oils from Biomass: Producing, Analyzing, and Upgrading; ACS Publications: Washington, DC, USA, 1988; pp. 179–188.
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061.
- Neveux, N.; Yuen, A.K.L.; Jazrawi, C.; Magnusson, M.; Haynes, B.S.; Masters, A.F. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341.
- Barreiro, D.L.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127.
- Li, B.; Magoua Mbeugang, C.F.; Xie, X.; Wei, J.; Zhang, S.; Zhang, L. Catalysis/CO2 sorption enhanced pyrolysis-gasification of biomass for H2-rich gas production: Effects of activated carbon, NiO active component and calcined dolomite. Fuel 2023, 334, 126842.
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Dutta, A. Simulation of biomass-plastic co-gasification in a fluidized bed reactor using Aspen plus. Fuel 2022, 319, 123708.
- Shahbaz, M.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew Sustain. Energy Rev. 2017, 73, 468–476.
- Sudhakar, K.; Rajesh, M.; Premalatha, M. A mathematical model to assess the potential of algal bio-fuels in India. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1114–1120.
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9.
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16.
- Nkemka, V.N.; Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manag. 2010, 91, 1573–1579.
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A. Biofuel production through micro- and macroalgae pyrolysis—A review of pyrolysis methods and process parameters. J. Anal. Appl. Pyrolysis 2019, 142, 104599.
This entry is offline, you can click here to edit this entry!
