Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
PIEZO1 is ubiquitously expressed in cells in different kinds of tissues throughout the body, which can sense physical or mechanical stimuli and translate them into intracellular electrochemical signals to regulate organism functions. In particular, PIEZO1 appears in complex interactive regulatory networks as a central node, governing normal and pathological functions in the body. PIEZO1 converts mechanical stimuli (strain) into biochemical signals, inducing alterations in protein conformation and activating intracellular biochemical signaling pathways.
- PIEZO1
- ion channel
- mechanotransduction
- glioma
- glia cell
1. Structure and Gating Mechanism of PIEZO1
With more than 2500 amino acids and a vast transmembrane domain, PIEZO1 is an evolutionarily conserved integral membrane protein that serves as a pore-forming subunit of the ion channel, but its structure and gating mechanism were not initially determined [1]. Due to the development of cryo-electron microscopy, live-cell immunoassays, and X-ray crystallography, its basic physical body has been identified and described as a homologous trimer assembled on a membrane with a helical blade-like structure [2][3]. It consists of a central cap, three peripheral blades, three long intracellular rays, an intracellular C-terminal domain (CTD), and a transmembrane (TM) fragment [2][3]. Based on the unique 38-TM structure inherent in its protomer, PIEZO1 can be divided into three functional components: 1. a mechano-sensing module for sensing mechanical stimuli, 2. a C-terminal ion-conducting pore module that acts as a channel, and 3. a transduction functional component for transmitting mechanical stimuli [4]. Each PIEZO1 protomer has an unprecedented structure of 38 TMs, of which the last 2 TMs at the C-terminus form an outer helix (OH) and an inner helix (IH), and the three IHs lock together to form a transmembrane pore [4][5]. The remaining TMs form abnormally curved, non-planar transmembrane helical blades that, all together, form nine tandemly repeating transmembrane helix units (THUs), where each unit consists of four TM fragments [5]. The partially non-planar TM blades form the central cap of the ion channel outside the cell, which is called the C-terminal extracellular structural domain (CED). The beam originates at the lower edge of the peripheral TM and physically connects the distal blade to the ion conduction pore module via a compound consisting of the CTD, anchor, and OH-IH. The specific structure of the beam reveals its dual role: transmembrane support and the transmission of conformational changes in extracellular blades [2][3]. The CTD, located above the proximal edge of the beam, interacts with the beam, and contains an anchor consisting of three alpha helices with a unique hairpin plane structure parallel to the film. The anchoring domain enables the formation of an inverted V-shape structure that penetrates the inner leaflet of the membrane, interacts with the IH and OH, and stabilizes the ion conduction pore [4].
It has been shown that PIEZO1 can be directly activated by the mechanical stretching of lipid bilayers [6][7]. The non-planar TM blades and THU structures form mechanoreceptors that directly sense minutiae changes in membrane curvature and tension [7][8]. The motion characteristics of the long rod-like beam and the peripheral THU are analogous to the lever motion, as the pivot on the beam near the central pore, consisting of residues L1342 and L1345, allows the PIEZO1 channel to efficiently translate the conformational changes of the extracellular blade into the opening and closing of the ion conduction pore, translating mechanical stimuli into chemical signals [5][9]. Based on the characteristic of PIEZO1 distorting the cytosolic membrane into a dome structure, some researchers have proposed the mechanism of “membrane doming” [10]. With the opening of the channel, the morphology of the membrane changes and the energy needed to modify the membrane is proportional to the change in the projected area under the dome [10]. This membrane doming mechanism explains the high mechanical sensitivity displayed by PIEZO1 through the ion selective pore.
Since membrane tension is largely dependent on the arrangement of the cytoskeleton and extracellular matrix (ECM), it is, therefore, reported that the cytoskeletal contractile protein myosin II stimulates PIEZO1 via the traction generated by myosin light-chain kinase (MLCK), and induces the change of the gate that triggers cation influx [11][12]. In addition, PIEZO1 also allows the interactions with a variety of proteins. These include myotubularin-related protein 2 (MTMR2) and stomatin-like protein3 (STOML3), which affect the activity of PIEZO1 channels according to cholesterol [13][14]. SERCA2 inhibits the PIEZO1 protein in a structural manner by binding to the anchored-OH junction of the linker pore module and the mechanical transduction module [15]. All in all, the PIEZO1 channel adopts both mechanotransduction and an ion channel to carry out its structural function in mechanosensing, channel opening, and ion conduction, which clarifies the channel mechanism based on mechanical force control.
2. Agonists and Antagonists
Many studies have investigated the ion conduction and mechanical gating mechanisms of the PIEZO1 channel. It has been reported that GsMTx4, an amphiphilic peptide toxin, may mechanically perturb PIEZO1 by modulating local membrane tension rather than directly acting on PIEZO1 itself [16]. Similarly, the amphiphilic macromolecule amyloid β (Aβ) can modulate channel activity by altering the membrane structure or cytoskeleton instead of working on it [17][18]. Ruthenium red (RR) works on the two residues with a negative charge in the intracellular CTD, blocking the ion conduction of PIEZO1 through a pore-plugging mechanism, while a series of TRPV channels other than the PIEZO1 channel are also inhibited by RR [1][9].
The cell membrane is composed of a lipid bilayer; saturated and polyunsaturated fatty acids can, therefore, regulate the activity of the PIEZO1 channel by adjusting membrane stiffness and lipids [19]. Compared with the antagonists of the PIEZO1 channels, Yoda1, the specific agonist of PIEZO1, is highly selective, acting exclusively on the variable binding domain of PIEZO1 [20]. As novel specifical activators of PIEZO1, Jedi1 and Jedi2 have been identified from more than 3000 compounds by using the fluorescent imaging plate technique as a calcium indicator [5]. Compared to Yoda1, Jedi1 and Jedi2 have higher water solubility, a faster onset current, and a shorter decay duration. More importantly, the joint application of Jedi1 and Yoda1 produces a synergistic effect of enhancing the inward current of PIEZO1 [5]. Recent studies have demonstrated that PIEZO1 is associated with various pathophysiological processes [21][22][23][24][25][26]. Drugs targeting it may, therefore, be promising for the treatment of many diseases [4][27][28][29]. However, the specific physiological mechanisms of its ligand-binding crystals still need to be further investigated since drugs that work on PIEZO1 lack robust specificity and potency [4]. Overall, with rapid progress in the elucidation of the structure and mechanism of PIEZO1, a growing number of agents are being developed for the treatment of PIEZO1 dysfunction-related diseases.
3. PIEZO1-Related Physiological and Pathological Processes
As an evolutionarily highly conserved protein, the PIEZO1 channel is expressed in a wide variety of cells that participate in different mechanotransduction processes under physiological and pathological conditions. The shear stress generated by the blood flow and membrane stretch caused by blood pressure fluctuations activates PIEZO1 channels embedded in the endothelium [30][31][32]. Several studies have shown that PIEZO1 regulates vascular remodeling, blood pressure, erythrocyte volume homeostasis, and lymphatic vessel development through Ca2+ influx or its interaction with integrin [31][32][33][34]. PIEZO1 has been shown to be overexpressed in osteoblasts as well as to affect the differentiation of osteoclasts and the resorption of bone by mediating the expression of type II and type IX collagen in osteoblasts that are governed by the YAP signaling pathway. In the case of PIEZO1 deletion, the osteoblast osteoclast crosstalk is out of balance, thus leading to the rapid loss of bone mass and causing spontaneous fractures [35][36].
Furthermore, the activation of PIEZO1 in myeloid cells initiates the effects of activator protein-1 (AP-1) and endothelin-1 (EDN1), thereby stabilizing the hypoxia-inducible factor 1α (HIF-1α) and inducing the expression of pro-inflammatory factors [37]. Another study suggests that toll-like receptor 4 (TLR4) works in synergy with PIEZO1 to strengthen the phagocytosis of macrophages to clear bacteria and resist the invasion of foreign substances [38]. Interestingly, PIEZO1 interacts with the classical inflammatory pathway JAK/STAT, the inflammasome NLPR3, the Ca2+-sensitive MAPK family, integrins, focal adhesion kinase (FAK), and calcium-dependent proteases to participate in the development and progression of inflammation [39][40][41][42][43][44][45][46][47][48]. PIEZO1 has been reported to be associated with iron metabolism in the organism, enhancing the ability of macrophages to engulf erythrocytes and inhibiting the expression of hepcidin, thereby leading to iron overload in the blood [49]. In radiation-injured endothelial cells of pulmonary microvascularization, PIEZO1 degrades cadherin in the vascular endothelium via calpain, causing an increase in ROS and the oxidation of lipids [50]. That is, PIEZO1 may also be involved in the regulation of endothelial cell ferroptosis.
In addition, PIEZO1 is overexpressed in a variety of tumors. The resting state of PIEZO1 has been reported to inhibit proliferation, migration, and invasion of tumor cells, and promote apoptosis of the cancer cells [21][39][51][52][53][54][55][56][57]. Mechanistically, the process is mediated mainly by an increase in the cytosolic calcium ion concentration, which initiates a series of relevant transductions of signals in the downstream, involving ERK1/2, AKT/mTOR, and YAP/TAZ [21][39][51][52][53][54][55][56][57]. To summarize, cells sense the mechanical stimuli via the PIEZO1 protein and then trigger calcium influx, which in turn triggers a cascade of downstream effects that ultimately induce conformational shifts of proteins and regulate gene expression, thus driving the cellular functions in physiological and pathological processes.
4. PIEZO1 in CNS Cells
Neurons and glial cells in the CNS exhibit significant mechanosensitivity, being capable of sensing mechanical stimuli from the ambient environment and converting them into biochemical signals to regulate their functions [6][23][58]. The critical value of PIEZO1 as a mechanosensor expressed in the membranes of the CNS is of growing interest, with an increasing number of studies aiming to discuss its role in neurological diseases [25][26][27][59]. As shown in Table 1, PIEZO1 is involved in the translation of mechanical signals in the CNS neurons and glial cells.
Table 1. Mechanotransduction of PIEZO1 in CNS cells.
| Cell Type | Mechanical Stimuli | State of PIEZO1 | Effects | References |
|---|---|---|---|---|
| Neuron | Ultrasound | activated | Elevated intracellular Ca2+ | [60] |
| activated | Initiating Ca2+ influx and affecting the levels of downstream Ca2+ signaling proteins involved in neuronal function | [61] | ||
| Substrate stiffness gradient | activated | Axonal growth and pathfinding errors | [62] | |
| Axon injury | activated | Inhibiting axon regeneration via the CamKII-Nos-PKG pathway | [63] | |
| Oxygen-glucose deprivation/reoxygenation injury | activated | Enhanced cell viability inhibition, apoptosis, increase intracellular calcium levels and enhanced calpain activity | [64] | |
| Microglial | Amyloid beta fibrils stiffness | activated | Inducing Ca2+ influx, phagocytosis and compacting of Aβ plaques | [65] |
| Osmotic pressure | activated | Increasing cytosolic Ca2+ signaling and regulate cell function via JNK1 and mTOR signaling pathway | [66] | |
| Astrocytes | Mechanical indentation stimulation | activated | Evoking Ca2+ response and ATP release as therefore regulates neurogenesis and cognitive functions | [67] |
| Oligodendrocyte progenitor cells | Mechanical stiffness gradient | inhibited | Increasing proliferation and differentiation | [68] |
| Neural stem cells | Stretch stress | activated | Directing the fate of the neural stem cells toward the desired lineage. | [69] |
Abbreviations: CamKII: calmodulin-dependent protein kinases; Nos: nitric oxide synthase; PKG: cGMP-dependent protein kinase G; JNK1: c-Jun N-terminal kinase 1; mTOR: mammalian target of rapamycin; ATP: adenosine triphosphate.
PIEZO1 is an essential ingredient in maintaining neuronal physiological function [62][63][69][70]. In a study measuring focal tissue stiffness in the brain at different developmental phases by observing brain tissue at different developmental nodes under an atomic force microscope, it was reported that PIEZO1 is fundamental in mentoring axonal growth and neurons’ maturation [62]. On the other hand, PIEZO1 channels can be activated to restrict nerve regeneration through the downstream cGMP kinase or PKG pathway in damaged neurons, triggering Ca2+ signaling [62][63][64]. Moreover, the mechanical activation of PIEZO1 with ultrasound is accompanied by an increase in Ca2+ influx and an elevated expression of activated calmodulin-dependent protein kinases (CaMKII) and the cAMP-response element binding protein (CREB) [61]. These two proteins are intimately implicated in neuronal plasticity, learning, and memory functions [61]. In addition, it is speculated that in the brain of those with Alzheimer’s disease (AD), astrocytes sense changes in the environment and subsequently relay the information to damaged neurons around the Aβ plaques instead of sensing stimuli via the damaged neurons that lost their PIEZO1 function [70]. Therefore, PIEZO1 expression is not detectable in the neurons in damaged regions of the AD brain. In contrast, PIEZO1 mRNA is detected in the neurons in both the non-AD brain and the non-damaged regions of the AD brain [70][71].
Astrocytes can take in and release a diversity of neuromodulatory signals for message delivery and account for the largest number of glial cells in the brain [72]. PIEZO1 is expressed in reactive astrocytes surrounding plaques in AD patients, whereas PIEZO1 mRNA is not detected in quiescent astrocytes, suggesting that PIEZO1 may be the expression gene in Aβ plaque-induced astrocytes [73]. Aging and infection can induce the upregulation of PIEZO1 in the plaque-induced reactive astrocytes, which may in turn trigger astrocyte proliferation [71]. In primary astrocytes derived from mice, some researchers use LPS to mimic the infection situation and observe an increase in PIEZO1 expression [74]. The upregulation of PIEZO1 elevates intracellular Ca2+ concentration while diminishing responsiveness of astrocytes to adenosine triphosphate (ATP), and inhibits the generation and release of pro-inflammatory cytokines and chemokines, ultimately suppressing neuroinflammation [74]. Moreover, evoked by external mechanical forces to generate cationic currents and Ca2+ signals, PIEZO1 in astrocytes also mediates spontaneous Ca2+ influx and triggers the release of ATP to regulate the development and neuronal maturation of neural stem cells (NSCs) [67]. In addition, the reduced volume of the hippocampal dentate gyrus in astrocyte-specific PIEZO1-deficient mice suggests that PIEZO1-mediated mechanotransduction affects the long-temporal enhancement (LTP) and the cognitive function of the hippocampus, consequently impairing learning and memory performance [67]. Consistent with this, the activity of PIEZO1 expressed on NSCs affects the definitive lineage selection of NSCs and guides their differentiation into either neurons or astrocytes [69]. Therefore, it is likely one of the mechanisms for the formation of astrocytes [69]. Collectively, the interaction between astrocytes and NSCs shows the significance of astrocytes as a center for intercellular message exchange in the CNS.
Under normal physiological conditions, the expression level of PIEZO1 in microglia is relatively high [75]. This may be because microglia, as the main residual immune cells in the brain, need to sense changes in the surrounding environment in time to perform immune functions for host defense [76]. Some studies have reported that any mechanical perturbation caused by intra- and extracellular osmotic pressure homeostasis or changes in environmental stiffness can activate PIEZO1 and provoke Ca2+ influx [65][66][75]. Elevated concentrations of intracellular Ca2+ interact with intracellular Ca2+-sensitive transcriptional regulators to regulate microglia proliferation and migration and to mediate neuroinflammation [65][75][77]. Immunofluorescence staining analysis of postmortem brain tissue sections from AD patients indicates that microglia cells cluster around Aβ plaques, and PIEZO1 protein levels are elevated [65]. In a recent study, it was proposed that microglia may sense the mechanical stimuli of plaques through PIEZO1 and enhance their own phagocytosis of foreign bodies, resulting in the cleavage, compaction, and later clearance of Aβ plaques [65]. Interestingly, the pathological effects of AD may modify the microglia membrane and cytoskeleton, impairing the PIEZO1-mediated Ca2+ signaling pathway and initiating downstream malfunction, which ultimately contributes to the inability of microglia to clear the Aβ amyloid plaques [75]. According to a new study using LPS to induce an inflammatory phenotype of microglia, the activation of the PIEZO1 channel is observed to inhibit the activation of microglia to exert anti-inflammatory effects, which suggests that microglia may regulate neuroinflammation via a novel mechanism involving PIEZO1 [78].
Recent studies have identified the expression of PIEZO1 as a key effector of sensing the stiffness of the matrix in oligodendrocyte progenitor cells (OPCs) in rodents [68]. The differentiation and proliferation of OPCs are stopped in harder substrates, whereas in softer substrates or those inhibiting the PIEZO1, this phenomenon can be reversed, suggesting that OPCs are PIEZO1 dependent during growth [68]. Similarly, the expression level of PIEZO1 in human MO3.13 oligodendrocytes changes at different stages of maturation. When PIEZO1 is suppressed by the antagonist GsMTx4, it induces the enhanced proliferation and migration of oligodendrocytes [79]. Further investigation reveals that the condition of oligodendrocytes is related to neurons, as neuronal axonopathy is often accompanied by the demyelination of oligodendrocytes and vice versa [80]. This can be explained by the overexpression of PIEZO1 channels in cortical neurons that leads to Ca2+ entering axons, triggering the release of more Ca2+ from the intracellular calcium reservoirs to activate the calpain-mediated demyelination [80].
Collectively, all these studies indicate that PIEZO1 mediates the glial-neuron interaction and plays a central role in the manifestations and pathological changes of brain function. Therefore, the specific mechanism needs to be clearly elucidated in future studies, as drugs that can target PIEZO1 for the neural regeneration and modulation of glial function have the potential to be used for the treatment of patients with brain diseases.
5. PIEZO1 in Gliomas
Gliomas, which originate from glial cells, are some of the most common brain tumors. The properties and behavior of the glial cells change during tumorigenesis [81][82]. Mechanical forces and biochemical signals control tumor formation and development during this process [81][82]. Strikingly, PIEZO1 physically localizes to the focal adhesion of glioma cells, catalyzing the maturation and growth of the focal adhesion through a force-dependent calcium signaling pathway [39][83]. Functional enrichment analysis of the China Glioma Genome Atlas (CGGA) dataset and the Gene Set Enrichment Analysis (GSEA) dataset revealed that PIEZO1 acts as a central node in a functional regulatory network that integrates regulators associated with tissue-stiffening molecules [53]. Consistent with this, RNA sequencing of PIEZO1 knockout glioblastoma cell lines and the TCGA database analysis for double-determining PIEZO1-related genes show that PIEZO1 is associated with the extracellular matrix (ECM), actin cytoskeleton remodeling, and the activation of integrin adhesion signaling [39]. In addition, the expression of PIEZO1 transduces mechanical stimuli among the glioma cells, promoting tumorigenesis and development [39]. This finding can explain how the changes in self-stiffness in gliomas are mainly caused by the pressure gradient generated by the tumor itself instead of collagen deposition or cross-linking [84]. The enhanced stiffness among glioma cells and the ECM microenvironment in turn regulates the activation of PIEZO1, facilitating the pathological process [39][83][84]. Moreover, many signaling pathways, including the matrix metalloproteinase (MMP) family, tissue inhibitors of the metalloproteinases (TIMP) family, the mitogen-activated protein kinase (MAPK) family, and the phosphoinositide 3-kinase (PI3K) family, are positively associated with the higher expression of PIEZO1 during the pathological process [53]. In short, an increased expression of PIEZO1 in most aggressive tumors, including glioblastoma, indicates a mechanical sensing and growth advantage of glioma cells.
Clinical studies on the expression of PIEZO1 in patients with gliomas demonstrate a similar tendency. The analysis of a clinical database that consists of 325 gliomas cases from the CGGA dataset and 276 cases from the GSE16011 cohort showed that the expression of PIEZO1 is highly correlated with the malignancy and molecular subtypes of gliomas [53]. Moreover, the overexpression of PIEZO1 contributes to more severe clinical symptoms, as was found via a retrospective analysis of imaging data and surgical samples from 64 patients with glioblastoma [85]. Additionally, immunohistochemical analysis of PIEZO1 in 183 patients with gliomas suggested that PIEZO1, as an independent factor, has an adverse impact on the prognosis of glioma patients [86]. The combination of the PIEZO1 expression level and WHO grade is much more accurate in predicting clinical outcomes [86]. Overall, PIEZO1 can be used as an indicator of glioma malignancy and is able to predict the clinical outcome in patients with gliomas.
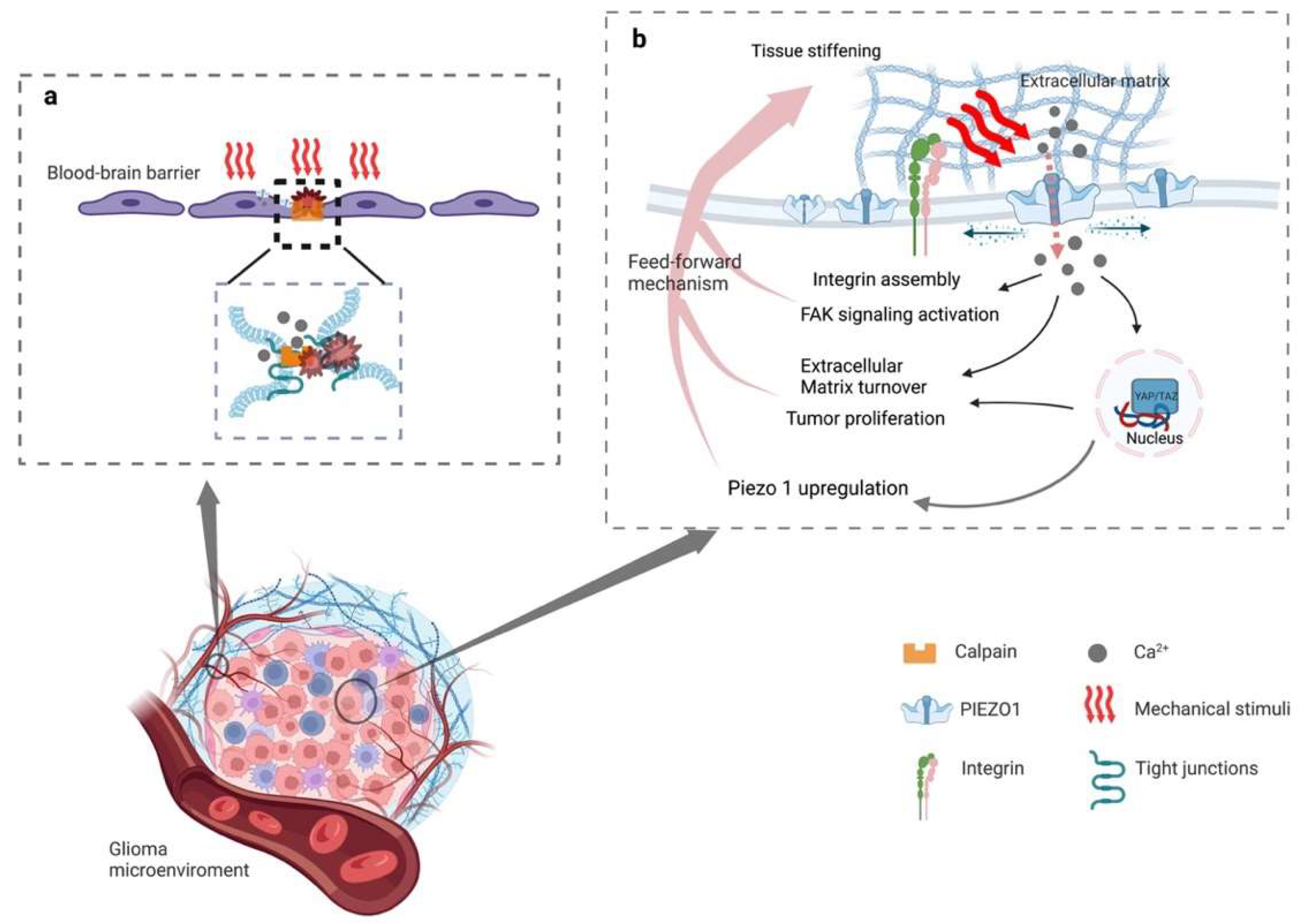
In addition, peritumoral brain edema (PTBE), including vasogenic and cytotoxic edema, exacerbates neurological signs and clinical symptoms in patients with gliomas, which can serve as an independent factor for predicting the prognosis and recurrence of the gliomas in such patients [85][87][88]. It has been postulated that vasogenic edema can be mediated through Ca2+ influx by opening the PIEZO1 ion channels, and then activating calpain and degrading the tight-junction protein between adjacent cells in glioblastomas (Figure 1a). There is, however, a lack of evidence for the further validation of this hypothesis [85]. Theoretically, cytotoxic edema happens due to extracellular cations entering neurons and glial cells through cation channels and accumulating in cells while cationic influx drives anions inflow [88]. The PIEZO1 ion channels may play a vital role during this process. However, no studies have yet supported the relationship between PIEZO1 and cytotoxic edema.

Figure 1. Schematic diagram of the mechanism of activated PIEZO1 in glioma progression and peritumoral edema. (a) Stiffening of the extracellular matrix activates PIEZO1, resulting in an influx of calcium. Intracellular elevated concentration of Ca2+ catalyzes the assembling and maturation of focal adhesion, and (in-)directly activates integrin-focal adhesion pathways that stimulate cell proliferation and regulate extracellular matrix remodeling. The increased expression of PIEZO1 reinforces the mechanosensory and mechanotransduction capacity of the tumor cells, generating a feedforward mechanism that promotes glioma progression. (b) Proliferating tumor cells compressing peripheral blood vessels and activating the PIEZO1 of endothelial causing calcium influx. The influx of calcium then reacts with calpain, which disrupts the tight junctions between the blood-brain barrier.
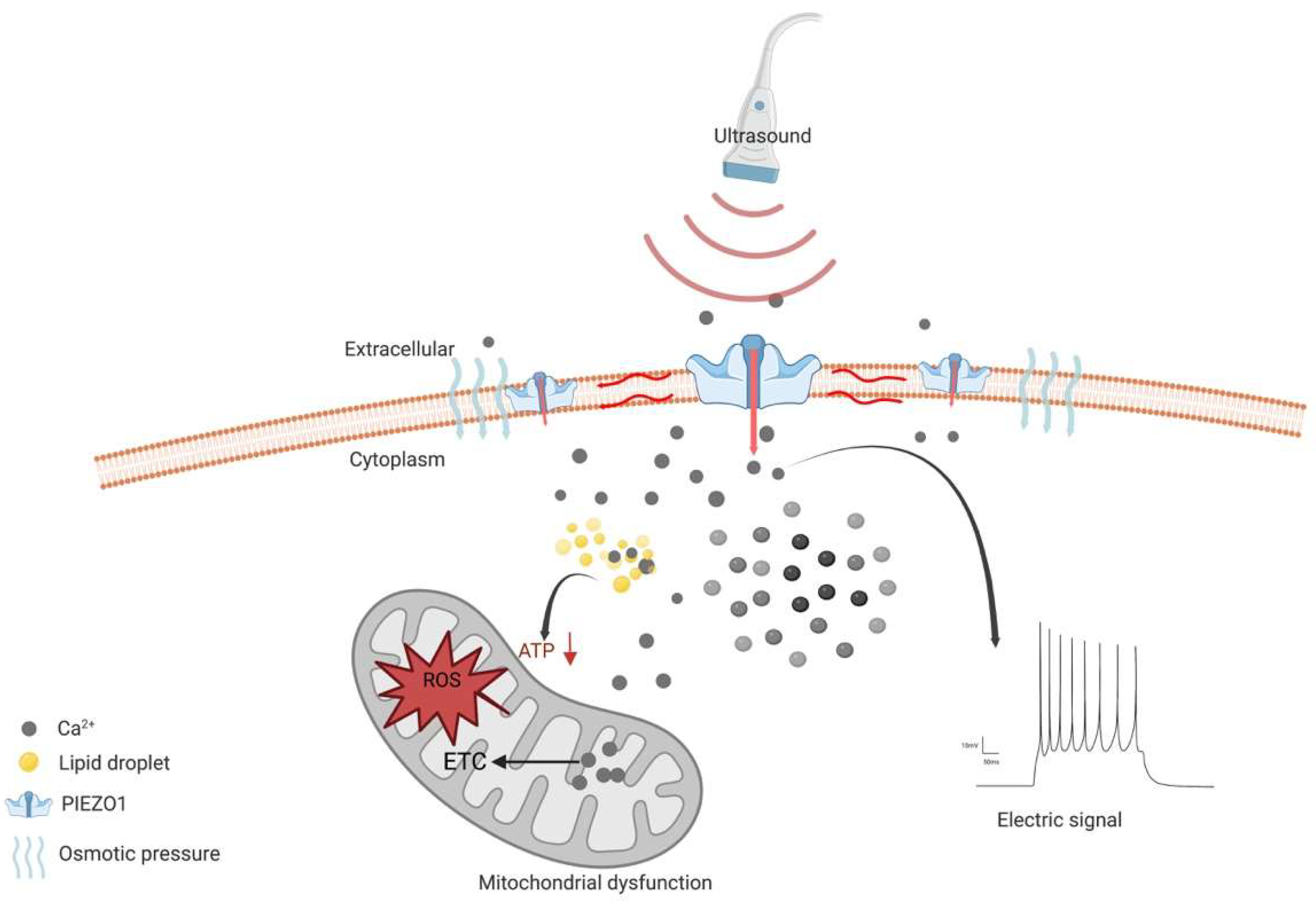
However, the emerging evidence shows that the activation of PIEZO1 may be a sonodynamic therapeutic target [89]. Since it helps transient Ca2+ influx combine with the lipid droplets, it forms a complex that disturbs the energy supply in gliomas in addition to cell swelling lead by the calcium pathway [89]. Together with previous studies, the opposite effect, led by the activation of PIEZO1, may be related to different mechanisms. On one hand, during glioma genesis, PIEZO1 gathers around focal adhesions, which activates regional calcium fluctuations and leads to adhesion maturation and cell polarization [83]. In addition, PIEZO1 interacts with integrin-dependent kinases (FAKs) and transmits signals to the transcriptional coactivator with a PDZ-binding motif (TAZ), leading to chromatin remodeling and changes in transcription levels (Figure 1b). On the other hand, as a therapeutic target in gliomas, PIEZO1 acts as an ion channel for translating mechanical stimuli to electrical and chemical signals. The mild calcium influx would change to a large intracellular calcium transient stimulated by ultrasound, leading to a drastic change in the voltage in the tumor cells (Figure 2).

Figure 2. Schematic diagram of the mechanism of PIEZO1 as an anti-glioma therapeutic target. PIEZO1 is activated by ultrasound and constantly opens, leading to Ca2+ influx and increased concentration of intracellular Ca2+. The osmotic pressure between intracellular and extracellular leads to cell swelling and induces cell death. High concentration of cytoplasmic Ca2+ enters mitochondria and elevates intra-mitochondria Ca2+, resulting in impaired electron transport chain (ETC) function. Subsequently, impaired ETC leads to the elevation of reactive oxygen species (ROS). All above are contributed to mitochondrial dysfunction, leading to cell death. In addition, the instantaneous opening of PIEZO1 leads to the rapid transmembrane of cations and a rapid increase in intra- and extracellular voltage difference.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15030883
References
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo Proteins Are Pore-Forming Subunits of Mechanically Activated Channels. Nature 2012, 483, 176–181.
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the Mammalian Mechanosensitive Piezo1 Channel. Nature 2015, 527, 64–69.
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.-Q.; et al. Structure and Mechanogating Mechanism of the Piezo1 Channel. Nature 2018, 554, 487–492.
- Xiao, B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 195–218.
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A Lever-like Transduction Pathway for Long-Distance Chemical- and Mechano-Gating of the Mechanosensitive Piezo1 Channel. Nat. Commun. 2018, 9, 1300.
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.-A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the Mechanoprotective Influence of the Cytoskeleton Reveals PIEZO1 Is Gated by Bilayer Tension. Nat. Commun. 2016, 7, 10366.
- Lewis, A.H.; Grandl, J. Mechanical Sensitivity of Piezo1 Ion Channels Can Be Tuned by Cellular Membrane Tension. eLife 2015, 4, e12088.
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the Mechanically Activated Ion Channel Piezo1. Nature 2018, 554, 481–486.
- Zhao, Q.; Wu, K.; Geng, J.; Chi, S.; Wang, Y.; Zhi, P.; Zhang, M.; Xiao, B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron 2016, 89, 1248–1263.
- Guo, Y.R.; MacKinnon, R. Structure-Based Membrane Dome Mechanism for Piezo Mechanosensitivity. eLife 2017, 6, e33660.
- Nourse, J.L.; Pathak, M.M. How Cells Channel Their Stress: Interplay between Piezo1 and the Cytoskeleton. Semin. Cell Dev. Biol. 2017, 71, 3–12.
- Ellefsen, K.L.; Holt, J.R.; Chang, A.C.; Nourse, J.L.; Arulmoli, J.; Mekhdjian, A.H.; Abuwarda, H.; Tombola, F.; Flanagan, L.A.; Dunn, A.R.; et al. Myosin-II Mediated Traction Forces Evoke Localized Piezo1-Dependent Ca2+ Flickers. Commun. Biol. 2019, 2, 1–13.
- Qi, Y.; Andolfi, L.; Frattini, F.; Mayer, F.; Lazzarino, M.; Hu, J. Membrane Stiffening by STOML3 Facilitates Mechanosensation in Sensory Neurons. Nat. Commun. 2015, 6, 8512.
- Narayanan, P.; Hütte, M.; Kudryasheva, G.; Taberner, F.J.; Lechner, S.G.; Rehfeldt, F.; Gomez-Varela, D.; Schmidt, M. Myotubularin Related Protein-2 and Its Phospholipid Substrate PIP2 Control Piezo2-Mediated Mechanotransduction in Peripheral Sensory Neurons. eLife 2018, 7, e32346.
- Zhang, T.; Chi, S.; Jiang, F.; Zhao, Q.; Xiao, B. A Protein Interaction Mechanism for Suppressing the Mechanosensitive Piezo Channels. Nat. Commun. 2017, 8, 1797.
- Gnanasambandam, R.; Ghatak, C.; Yasmann, A.; Nishizawa, K.; Sachs, F.; Ladokhin, A.S.; Sukharev, S.I.; Suchyna, T.M. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys. J. 2017, 112, 31–45.
- Maneshi, M.M.; Ziegler, L.; Sachs, F.; Hua, S.Z.; Gottlieb, P.A. Enantiomeric Aβ Peptides Inhibit the Fluid Shear Stress Response of PIEZO1. Sci. Rep. 2018, 8, 14267.
- Cox, C.D.; Gottlieb, P.A. Amphipathic Molecules Modulate PIEZO1 Activity. Biochem. Soc. Trans. 2019, 47, 1833–1842.
- Romero, L.O.; Massey, A.E.; Mata-Daboin, A.D.; Sierra-Valdez, F.J.; Chauhan, S.C.; Cordero-Morales, J.F.; Vásquez, V. Dietary Fatty Acids Fine-Tune Piezo1 Mechanical Response. Nat. Commun. 2019, 10, 1200.
- Botello-Smith, W.M.; Jiang, W.; Zhang, H.; Ozkan, A.D.; Lin, Y.-C.; Pham, C.N.; Lacroix, J.J.; Luo, Y. A Mechanism for the Activation of the Mechanosensitive Piezo1 Channel by the Small Molecule Yoda1. Nat. Commun. 2019, 10, 4503.
- Yu, J.-L.; Liao, H.-Y. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1) in Human Cancer. Biomed. Pharmacother. 2021, 140, 111692.
- Wang, Y.; Xiao, B. The Mechanosensitive Piezo1 Channel: Structural Features and Molecular Bases Underlying Its Ion Permeation and Mechanotransduction. J. Physiol. 2018, 596, 969–978.
- Tortorella, I.; Argentati, C.; Emiliani, C.; Morena, F.; Martino, S. Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis. Cells 2022, 11, 3093.
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 2022, 13, 11.
- Lai, A.; Cox, C.D.; Chandra Sekar, N.; Thurgood, P.; Jaworowski, A.; Peter, K.; Baratchi, S. Mechanosensing by Piezo1 and Its Implications for Physiology and Various Pathologies. Biol. Rev. 2022, 97, 604–614.
- Syeda, R. Physiology and Pathophysiology of Mechanically Activated PIEZO Channels. Annu. Rev. Neurosci. 2021, 44, 383–402.
- Bryniarska-Kubiak, N.; Kubiak, A.; Lekka, M.; Basta-Kaim, A. The Emerging Role of Mechanical and Topographical Factors in the Development and Treatment of Nervous System Disorders: Dark and Light Sides of the Force. Pharmacol. Rep. 2021, 73, 1626–1641.
- Bryniarska-Kubiak, N.; Kubiak, A.; Basta-Kaim, A. Mechanotransductive Receptor Piezo1 as a Promising Target in the Treatment of Neurological Diseases. Curr. Neuropharmacol. 2022.
- Braidotti, N.; Chen, S.N.; Long, C.S.; Cojoc, D.; Sbaizero, O. Piezo1 Channel as a Potential Target for Hindering Cardiac Fibrotic Remodeling. Int. J. Mol. Sci. 2022, 23, 8065.
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 Integration of Vascular Architecture with Physiological Force. Nature 2014, 515, 279–282.
- Romac, J.M.-J.; Shahid, R.A.; Swain, S.M.; Vigna, S.R.; Liddle, R.A. Piezo1 Is a Mechanically Activated Ion Channel and Mediates Pressure Induced Pancreatitis. Nat. Commun. 2018, 9, 1715.
- Liu, T.; Du, X.; Zhang, B.; Zi, H.; Yan, Y.; Yin, J.; Hou, H.; Gu, S.; Chen, Q.; Du, J. Piezo1-Mediated Ca2+ Activities Regulate Brain Vascular Pathfinding during Development. Neuron 2020, 108, 180–192.e5.
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 Mediates Angiogenesis through Activation of MT1-MMP Signaling. Am. J. Physiol. Cell Physiol. 2019, 316, C92–C103.
- Nonomura, K.; Lukacs, V.; Sweet, D.T.; Goddard, L.M.; Kanie, A.; Whitwam, T.; Ranade, S.S.; Fujimori, T.; Kahn, M.L.; Patapoutian, A. Mechanically Activated Ion Channel PIEZO1 Is Required for Lymphatic Valve Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 12817–12822.
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by Mechanical Signals Promotes Bone Anabolism. eLife 2019, 8, e49631.
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical Sensing Protein PIEZO1 Regulates Bone Homeostasis via Osteoblast-Osteoclast Crosstalk. Nat. Commun. 2020, 11, 282.
- Tolar, P.; Wack, A. Monocytes Work Harder under Pressure. Nat. Immunol. 2019, 20, 1422–1424.
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 Signalling via Piezo1 Engages and Enhances the Macrophage Mediated Host Response during Bacterial Infection. Nat. Commun. 2021, 12, 3519.
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.-K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e7.
- Mechanically Activated Piezo1 Channels of Cardiac Fibroblasts Stimulate P38 Mitogen-Activated Protein Kinase Activity and Interleukin-6 Secretion—Journal of Biological Chemistry. Available online: https://www.jbc.org/article/S0021-9258(20)30734-1/fulltext (accessed on 5 December 2022).
- Sun, Y.; Leng, P.; Song, M.; Li, D.; Guo, P.; Xu, X.; Gao, H.; Li, Z.; Li, C.; Zhang, H. Piezo1 Activates the NLRP3 Inflammasome in Nucleus Pulposus Cell-Mediated by Ca2+/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 85, 106681.
- Lohberger, B.; Kaltenegger, H.; Weigl, L.; Mann, A.; Kullich, W.; Stuendl, N.; Leithner, A.; Steinecker-Frohnwieser, B. Mechanical Exposure and Diacerein Treatment Modulates Integrin-FAK-MAPKs Mechanotransduction in Human Osteoarthritis Chondrocytes. Cell. Signal. 2019, 56, 23–30.
- Liu, S.; Xu, X.; Fang, Z.; Ning, Y.; Deng, B.; Pan, X.; He, Y.; Yang, Z.; Huang, K.; Li, J. Piezo1 Impairs Hepatocellular Tumor Growth via Deregulation of the MAPK-Mediated YAP Signaling Pathway. Cell Calcium 2021, 95, 102367.
- Jin, Y.; Li, J.; Wang, Y.; Ye, R.; Feng, X.; Jing, Z.; Zhao, Z. Functional Role of Mechanosensitive Ion Channel Piezo1 in Human Periodontal Ligament Cells. Angle Orthod. 2015, 85, 87–94.
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258.
- Emig, R.; Knodt, W.; Krussig, M.J.; Zgierski-Johnston, C.M.; Gorka, O.; Groß, O.; Kohl, P.; Ravens, U.; Peyronnet, R. Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing. Cells 2021, 10, 663.
- Albarrán-Juárez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 Promote Endothelial Inflammation Depending on Flow Pattern and Integrin Activation. J. Exp. Med. 2018, 215, 2655–2672.
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin Activation by Fam38A Uses a Novel Mechanism of R-Ras Targeting to the Endoplasmic Reticulum. J. Cell Sci. 2010, 123, 51–61.
- Ma, S.; Dubin, A.E.; Zhang, Y.; Mousavi, S.A.R.; Wang, Y.; Coombs, A.M.; Loud, M.; Andolfo, I.; Patapoutian, A. A Role of PIEZO1 in Iron Metabolism in Mice and Humans. Cell 2021, 184, 969–982.e13.
- Guo, X.-W.; Zhang, H.; Huang, J.-Q.; Wang, S.-N.; Lu, Y.; Cheng, B.; Dong, S.-H.; Wang, Y.-Y.; Li, F.-S.; Li, Y.-W. PIEZO1 Ion Channel Mediates Ionizing Radiation-Induced Pulmonary Endothelial Cell Ferroptosis via Ca2+/Calpain/VE-Cadherin Signaling. Front. Mol. Biosci. 2021, 8, 725274.
- Zhao, F.; Zhang, L.; Wei, M.; Duan, W.; Wu, S.; Kasim, V. Mechanosensitive Ion Channel PIEZO1 Signaling in the Hall-Marks of Cancer: Structure and Functions. Cancers 2022, 14, 4955.
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on Altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780.
- Zhou, W.; Liu, X.; van Wijnbergen, J.W.M.; Yuan, L.; Liu, Y.; Zhang, C.; Jia, W. Identification of PIEZO1 as a Potential Prognostic Marker in Gliomas. Sci. Rep. 2020, 10, 16121.
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive Ion Channel Piezo1 Promotes Prostate Cancer Development through the Activation of the Akt/MTOR Pathway and Acceleration of Cell Cycle. Int. J. Oncol. 2019, 55, 629–644.
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical Stretch Triggers Rapid Epithelial Cell Division through Piezo1. Nature 2017, 543, 118–121.
- Shen, Y.; Pan, Y.; Guo, S.; Sun, L.; Zhang, C.; Wang, L. The Roles of Mechanosensitive Ion Channels and Associated Downstream MAPK Signaling Pathways in PDLC Mechanotransduction. Mol. Med. Rep. 2020, 21, 2113–2122.
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP Signaling Induces PIEZO1 to Promote Oral Squamous Cell Carcinoma Cell Proliferation. J. Pathol. 2021, 253, 80–93.
- Tyler, W. The Mechanobiology of Brain Function. Nat. Rev. Neurosci. 2012, 13, 867–878.
- Harraz, O.F.; Klug, N.R.; Senatore, A.J.; Hill-Eubanks, D.C.; Nelson, M.T. Piezo1 Is a Mechanosensor Channel in Central Nervous System Capillaries. Circ. Res. 2022, 130, 1531–1546.
- Shen, X.; Song, Z.; Xu, E.; Zhou, J.; Yan, F. Sensitization of Nerve Cells to Ultrasound Stimulation through Piezo1-Targeted Microbubbles. Ultrason. Sonochem. 2021, 73, 105494.
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. IScience 2019, 21, 448–457.
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; da Costa, L.F.; Guck, J.; et al. Mechanosensing Is Critical for Axon Growth in the Developing Brain. Nat. Neurosci. 2016, 19, 1592–1598.
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389.e6.
- Wang, Y.-Y.; Zhang, H.; Ma, T.; Lu, Y.; Xie, H.-Y.; Wang, W.; Ma, Y.-H.; Li, G.-H.; Li, Y.-W. Piezo1 Mediates Neuron Oxygen-Glucose Deprivation/Reoxygenation Injury via Ca2+/Calpain Signaling. Biochem. Biophys. Res. Commun. 2019, 513, 147–153.
- Hu, J.; Chen, Q.; Zhu, H.; Hou, L.; Liu, W.; Yang, Q.; Shen, H.; Chai, G.; Zhang, B.; Chen, S.; et al. Microglial Piezo1 Senses Aβ Fibril Stiffness to Restrict Alzheimer’s Disease. Neuron 2022, 111, 15–29.
- Liu, H.; Bian, W.; Yang, D.; Yang, M.; Luo, H. Inhibiting the Piezo1 Channel Protects Microglia from Acute Hyperglycaemia Damage through the JNK1 and MTOR Signalling Pathways. Life Sci. 2021, 264, 118667.
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-Mediated Mechanotransduction Determines Adult Neurogenesis and Cognitive Functions. Neuron 2022, 110, 2984–2999.e8.
- Segel, M.; Neumann, B.; Hill, M.F.E.; Weber, I.P.; Viscomi, C.; Zhao, C.; Young, A.; Agley, C.C.; Thompson, A.J.; Gonzalez, G.A.; et al. Niche Stiffness Underlies the Ageing of Central Nervous System Progenitor Cells. Nature 2019, 573, 130–134.
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-Activated Ion Channel Piezo1 Directs Lineage Choice in Human Neural Stem Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153.
- Blumenthal, N.R.; Hermanson, O.; Heimrich, B.; Shastri, V.P. Stochastic Nanoroughness Modulates Neuron–Astrocyte Interactions and Function via Mechanosensing Cation Channels. Proc. Natl. Acad. Sci. USA 2014, 111, 16124–16129.
- Velasco-Estevez, M.; Mampay, M.; Boutin, H.; Chaney, A.; Warn, P.; Sharp, A.; Burgess, E.; Moeendarbary, E.; Dev, K.K.; Sheridan, G.K. Infection Augments Expression of Mechanosensing Piezo1 Channels in Amyloid Plaque-Reactive Astrocytes. Front. Aging Neurosci. 2018, 10, 332.
- Semyanov, A.; Verkhratsky, A. Astrocytic Processes: From Tripartite Synapses to the Active Milieu. Trends Neurosci. 2021, 44, 781–792.
- Satoh, K.; Hata, M.; Takahara, S.; Tsuzaki, H.; Yokota, H.; Akatsu, H.; Yamamoto, T.; Kosaka, K.; Yamada, T. A Novel Membrane Protein, Encoded by the Gene Covering KIAA0233, Is Transcriptionally Induced in Senile Plaque-Associated Astrocytes. Brain Res. 2006, 1108, 19–27.
- Velasco-Estevez, M.; Rolle, S.O.; Mampay, M.; Dev, K.K.; Sheridan, G.K. Piezo1 Regulates Calcium Oscillations and Cytokine Release from Astrocytes. Glia 2020, 68, 145–160.
- Jäntti, H.; Sitnikova, V.; Ishchenko, Y.; Shakirzyanova, A.; Giudice, L.; Ugidos, I.F.; Gómez-Budia, M.; Korvenlaita, N.; Ohtonen, S.; Belaya, I.; et al. Microglial Amyloid Beta Clearance Is Driven by PIEZO1 Channels. J. Neuroinflammation 2022, 19, 147.
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369.
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483.
- Malko, P.; Jia, X.; Wood, I.; Jiang, L. Piezo1 Channel-Mediated Ca2+ Signaling Inhibits Lipopolysaccharide-Induced Activation of the NF-ΚB Inflammatory Signaling Pathway and Generation of TNF-α and IL-6 in Microglial Cells. Glia 2022.
- Velasco-Estevez, M.; Koch, N.; Klejbor, I.; Caratis, F.; Rutkowska, A. Mechanoreceptor Piezo1 Is Downregulated in Multiple Sclerosis Brain and Is Involved in the Maturation and Migration of Oligodendrocytes In Vitro. Front. Cell. Neurosci. 2022, 16, 914985.
- Velasco-Estevez, M.; Gadalla, K.K.E.; Liñan-Barba, N.; Cobb, S.; Dev, K.K.; Sheridan, G.K. Inhibition of Piezo1 Attenuates Demyelination in the Central Nervous System. Glia 2020, 68, 356–375.
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Prim. 2015, 1, 1–18.
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820.
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Tran Van Nhieu, G.; Cox, C.D.; Sheetz, M. Force- and Cell State–Dependent Recruitment of Piezo1 Drives Focal Adhesion Dynamics and Calcium Entry. Sci. Adv. 2022, 8, eabo1461.
- Pogoda, K.; Chin, L.; Georges, P.C.; Byfield, F.J.; Bucki, R.; Kim, R.; Weaver, M.; Wells, R.G.; Marcinkiewicz, C.; Janmey, P.A. Compression Stiffening of Brain and Its Effect on Mechanosensing by Glioma Cells. New J. Phys. 2014, 16, 075002.
- Qu, S.; Hu, T.; Qiu, O.; Su, Y.; Gu, J.; Xia, Z. Effect of Piezo1 Overexpression on Peritumoral Brain Edema in Glioblastomas. Am. J. Neuroradiol. 2020, 41, 1423–1429.
- Qu, S.; Li, S.; Hu, Z. Upregulation of Piezo1 Is a Novel Prognostic Indicator in Glioma Patients. Cancer Manag. Res. 2020, 12, 3527–3536.
- Schoenegger, K.; Oberndorfer, S.; Wuschitz, B.; Struhal, W.; Hainfellner, J.; Prayer, D.; Heinzl, H.; Lahrmann, H.; Marosi, C.; Grisold, W. Peritumoral Edema on MRI at Initial Diagnosis: An Independent Prognostic Factor for Glioblastoma? Eur. J. Neurol. 2009, 16, 874–878.
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic Edema: Mechanisms of Pathological Cell Swelling. Neurosurg. Focus 2007, 22, 1–9.
- Chen, L.; Yan, Y.; Kong, F.; Wang, J.; Zeng, J.; Fang, Z.; Wang, Z.; Liu, Z.; Liu, F. Contribution of Oxidative Stress Induced by Sonodynamic Therapy to the Calcium Homeostasis Imbalance Enhances Macrophage Infiltration in Glioma Cells. Cancers 2022, 14, 2036.
This entry is offline, you can click here to edit this entry!
